当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photo-induced oxidant-free oxidative C-H/N-H cross-coupling between arenes and azoles.
Nature Communications ( IF 14.7 ) Pub Date : 2017-02-01 , DOI: 10.1038/ncomms14226 Linbin Niu , Hong Yi , Shengchun Wang , Tianyi Liu , Jiamei Liu , Aiwen Lei
Nature Communications ( IF 14.7 ) Pub Date : 2017-02-01 , DOI: 10.1038/ncomms14226 Linbin Niu , Hong Yi , Shengchun Wang , Tianyi Liu , Jiamei Liu , Aiwen Lei
|
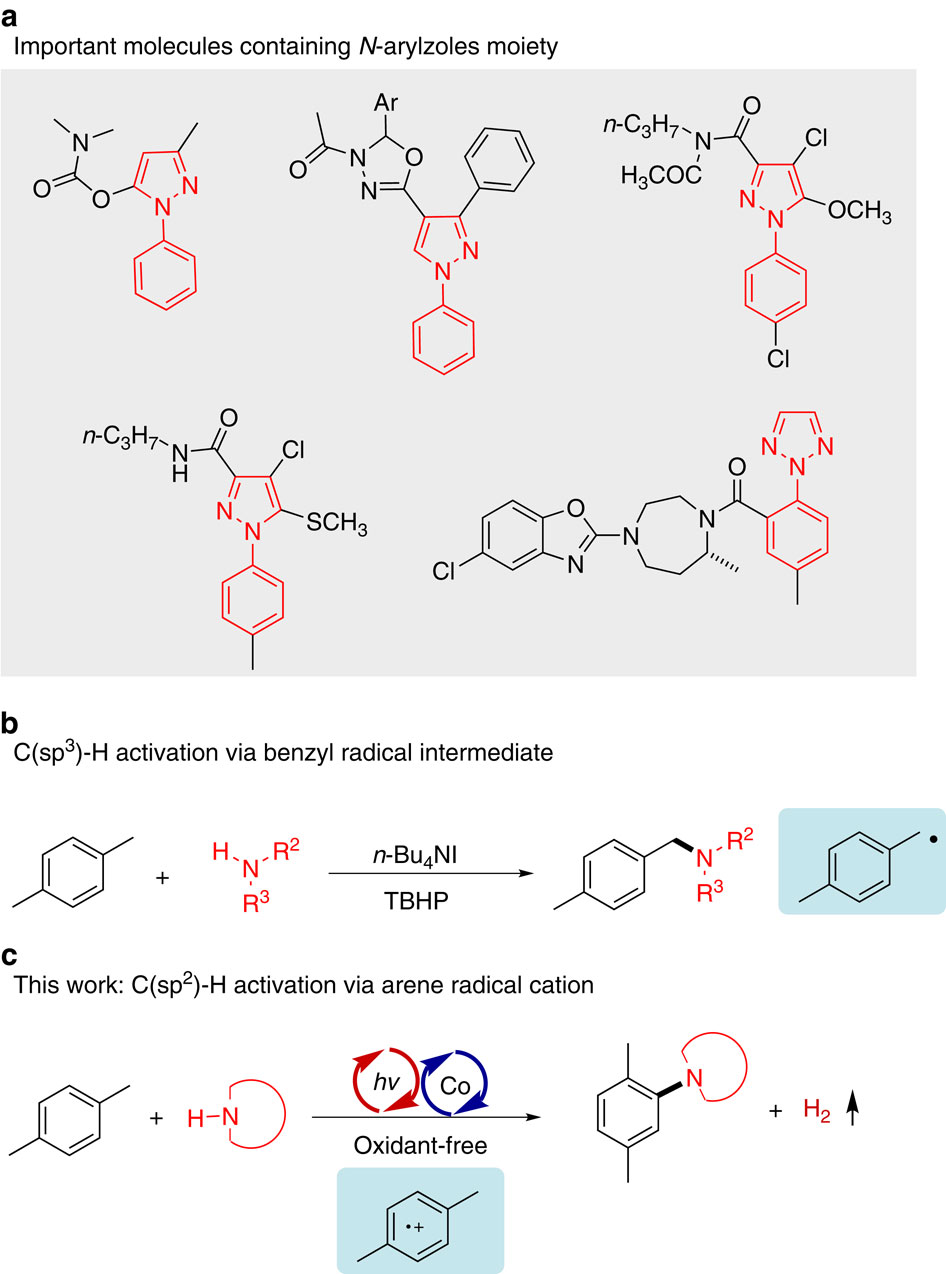
Direct cross-coupling between simple arenes and heterocyclic amines under mild conditions is undoubtedly important for C-N bonds construction. Selective C(sp2)-H amination is more valuable. Herein we show a selective C(sp2)-H amination of arenes (alkyl-substituted benzenes, biphenyl and anisole derivatives) accompanied by hydrogen evolution by using heterocyclic azoles as nitrogen sources. The reaction is selective for C(sp2)-H bonds, providing a mild route to N-arylazoles. The KIE (kinetic isotope effect) experiment reveals the cleavage of C-H bond is not involved in the rate-determining step. Kinetic studies indicate the first-order behaviour with respect to the arene component. It is interesting that this system works without the need for any sacrificial oxidant and is highly selective for C(sp2)-H activation, whereas C(sp3)-H bonds are unaffected. This study may have significant implications for the functionalization of methylarenes which are sensitive to oxidative conditions.
中文翻译:

芳烃和唑之间的光诱导无氧化剂氧化CH / NH交叉偶联。
在温和条件下,简单的芳烃与杂环胺之间的直接交叉偶联无疑对CN键的构建很重要。选择性C(sp 2)-H胺化更有价值。本文中,我们显示了使用杂环唑类作为氮源时,芳烃(烷基取代的苯,联苯和苯甲醚衍生物)的选择性C(sp 2)-H胺化反应,伴随着氢气的逸出。该反应对C(sp 2)-H键,提供了通向N-芳基唑的温和途径。KIE(动力学同位素效应)实验表明,速率确定步骤中不涉及CH键的裂解。动力学研究表明有关芳烃成分的一阶行为。有趣的是,该系统无需任何牺牲氧化剂即可工作,并且对C(sp 2)-H活化具有高度选择性,而C(sp 3)-H键不受影响。这项研究可能对对氧化条件敏感的甲基芳烃的功能化具有重要意义。
更新日期:2017-02-02
中文翻译:

芳烃和唑之间的光诱导无氧化剂氧化CH / NH交叉偶联。
在温和条件下,简单的芳烃与杂环胺之间的直接交叉偶联无疑对CN键的构建很重要。选择性C(sp 2)-H胺化更有价值。本文中,我们显示了使用杂环唑类作为氮源时,芳烃(烷基取代的苯,联苯和苯甲醚衍生物)的选择性C(sp 2)-H胺化反应,伴随着氢气的逸出。该反应对C(sp 2)-H键,提供了通向N-芳基唑的温和途径。KIE(动力学同位素效应)实验表明,速率确定步骤中不涉及CH键的裂解。动力学研究表明有关芳烃成分的一阶行为。有趣的是,该系统无需任何牺牲氧化剂即可工作,并且对C(sp 2)-H活化具有高度选择性,而C(sp 3)-H键不受影响。这项研究可能对对氧化条件敏感的甲基芳烃的功能化具有重要意义。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号