当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of binding modes and binding constants for the complexes of 6H-pyrido[4,3-b]carbazole derivatives with DNA
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-01-31 16:00:19 Akihito Shimazu, Masashi Kawagoshi, Shoichi Takeda, Haruaki Kurasaki, Asako Kato, Nahoko Morii, Norio Sakai, Takeo Konakahara
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-01-31 16:00:19 Akihito Shimazu, Masashi Kawagoshi, Shoichi Takeda, Haruaki Kurasaki, Asako Kato, Nahoko Morii, Norio Sakai, Takeo Konakahara
|
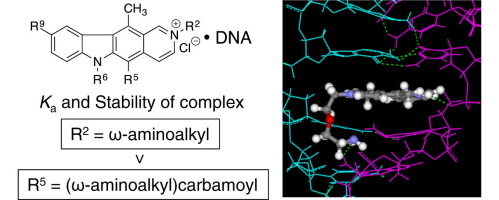
The binding modes and binding constants for the complexes of forty types of pyridocarbazole derivatives 1–40 with double stranded DNAs (dsDNAs) were reported. The binding modes were determined by a combination of a deflection spectroscopy and orientation of the corresponding molecule in the DNA-based film with chain alignment. All of the compounds exhibited the intercalation-binding mode. Its binding constants K a for the complexes, determined by quartz crystal microbalance (QCM), varied from 1.7×105 to 4.5×107 M−1 according to the substituents on the pyridocarbazole framework and the sequences of dsDNA. The binding constants K a of pyridocarbazole derivatives possessing the 2-(ω-amino)alkyl group and 5-(ω-amino)alkylcarbamyl group were larger than those of the corresponding ω-ureido derivatives. These ω-amino compounds exhibited strong GC base-pair preference in complexation. The K a values decreased with the increasing NaCl concentration. It was clarified by a molecular modeling that the framework of the 2-tethered ω-amino derivative was completely overlapped with the stacking GC base-pairs leading to the formation of the stable intercalative-complex, and that the framework of the 5-tethered ureido derivative was half overlapped leading to the formation of the unstable complex. Furthermore, there were good linear relationships between ln K a and the relative stabilities S rel of the complexes. Contrary to our expectation, there was no linear relationship between ln K a and IC50 against Sarcoma-180, NIH3T3, and HeLa S-3 cell lines.
中文翻译:

6H-吡啶并[4,3-b]咔唑衍生物与DNA配合物的结合方式和结合常数的测定
报道了40种类型的吡啶并咔唑衍生物1–40与双链DNA(dsDNA)的配合物的结合模式和结合常数。通过偏转光谱和基于DNA的膜中相应分子的取向与链排列的组合来确定结合模式。所有化合物均表现出插层结合模式。根据吡啶并咔唑骨架上的取代基和dsDNA的序列,通过石英晶体微量天平(QCM)测定的其对于络合物的结合常数K a从1.7×10 5到4.5×10 7 M -1变化。结合常数K a具有2-(ω-氨基)烷基和5-(ω-氨基)烷基氨基甲酰基的吡啶并咔唑衍生物的“α-羟基”大于相应的ω-脲基衍生物的β-羟基。这些ω-氨基化合物在络合中表现出强烈的GC碱基对偏好。随着NaCl浓度的增加,K a值降低。通过分子模型阐明,2系ω-氨基衍生物的骨架与堆积的GC碱基对完全重叠,从而导致形成稳定的嵌入复合物,以及5系脲基的骨架衍生物半重叠导致形成不稳定的络合物。此外,ln K a与相对稳定性S rel之间存在良好的线性关系。的复合体。与我们的预期相反,ln K a和IC 50与肉瘤180,NIH3T3和HeLa S-3细胞系之间没有线性关系。
更新日期:2017-02-01
中文翻译:

6H-吡啶并[4,3-b]咔唑衍生物与DNA配合物的结合方式和结合常数的测定
报道了40种类型的吡啶并咔唑衍生物1–40与双链DNA(dsDNA)的配合物的结合模式和结合常数。通过偏转光谱和基于DNA的膜中相应分子的取向与链排列的组合来确定结合模式。所有化合物均表现出插层结合模式。根据吡啶并咔唑骨架上的取代基和dsDNA的序列,通过石英晶体微量天平(QCM)测定的其对于络合物的结合常数K a从1.7×10 5到4.5×10 7 M -1变化。结合常数K a具有2-(ω-氨基)烷基和5-(ω-氨基)烷基氨基甲酰基的吡啶并咔唑衍生物的“α-羟基”大于相应的ω-脲基衍生物的β-羟基。这些ω-氨基化合物在络合中表现出强烈的GC碱基对偏好。随着NaCl浓度的增加,K a值降低。通过分子模型阐明,2系ω-氨基衍生物的骨架与堆积的GC碱基对完全重叠,从而导致形成稳定的嵌入复合物,以及5系脲基的骨架衍生物半重叠导致形成不稳定的络合物。此外,ln K a与相对稳定性S rel之间存在良好的线性关系。的复合体。与我们的预期相反,ln K a和IC 50与肉瘤180,NIH3T3和HeLa S-3细胞系之间没有线性关系。






























 京公网安备 11010802027423号
京公网安备 11010802027423号