当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of (E)-3,4-dihydroxystyryl 4-acylaminophenethyl sulfone, sulfoxide derivatives as dual inhibitors of HIV-1 CCR5 and integrase
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-01-31 16:00:19 Yixing Sun, Weisi Xu, Ningning Fan, Xuefeng Sun, Xianling Ning, Liying Ma, Junyi Liu, Xiaowei Wang
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-01-31 16:00:19 Yixing Sun, Weisi Xu, Ningning Fan, Xuefeng Sun, Xianling Ning, Liying Ma, Junyi Liu, Xiaowei Wang
|
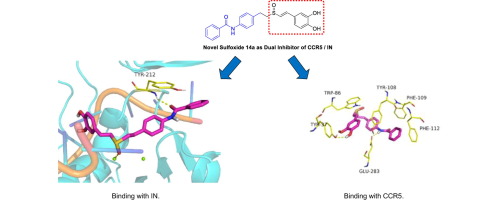
Aiming at the limited effectiveness of current clinical therapeutic effect of AIDS, novel series of compounds bearing (E)-3,4-dihydroxystyryl sulfone (or sulfoxide) and anilide fragments were designed and synthesized as dual inhibitors of HIV-1 CCR5/IN. The biological results indicated that several target compounds showed inhibitory activity against HIV-1 Bal (R5) infection in TZM-bl cells. Besides targeting the chemokine receptor on the host cell surface, they also displayed binding affinities with HIV-1 integrase using the surface plasmon resonance (SPR) binding assays. Molecular docking studies have inferred the possible binding mode of target compounds against integrase. These data demonstrate that the structure of (E)-3,4-dihydroxystyryl sulfone and sulfoxide derivatives have the potential to derive potent dual inhibitors of HIV-1 Integrase and CCR5.
中文翻译:

设计,合成和生物学评价(E)-3,4-二羟基苯乙烯基4-酰基氨基苯乙基砜,亚砜衍生物作为HIV-1 CCR5和整合酶的双重抑制剂
针对目前艾滋病临床治疗效果有限的情况,设计并合成了一系列新的带有(E)-3,4-二羟基苯乙烯基砜(或亚砜)和苯胺化物片段的化合物,作为HIV-1 CCR5 / IN的双重抑制剂。生物学结果表明,几种目标化合物在TZM-b1细胞中显示出对HIV-1 Bal(R5)感染的抑制活性。除了靶向宿主细胞表面的趋化因子受体外,他们还使用表面等离振子共振(SPR)结合测定法显示了与HIV-1整合酶的结合亲和力。分子对接研究已经推断出目标化合物针对整合酶的可能结合方式。这些数据表明(E)-3的结构
更新日期:2017-02-01
中文翻译:

设计,合成和生物学评价(E)-3,4-二羟基苯乙烯基4-酰基氨基苯乙基砜,亚砜衍生物作为HIV-1 CCR5和整合酶的双重抑制剂
针对目前艾滋病临床治疗效果有限的情况,设计并合成了一系列新的带有(E)-3,4-二羟基苯乙烯基砜(或亚砜)和苯胺化物片段的化合物,作为HIV-1 CCR5 / IN的双重抑制剂。生物学结果表明,几种目标化合物在TZM-b1细胞中显示出对HIV-1 Bal(R5)感染的抑制活性。除了靶向宿主细胞表面的趋化因子受体外,他们还使用表面等离振子共振(SPR)结合测定法显示了与HIV-1整合酶的结合亲和力。分子对接研究已经推断出目标化合物针对整合酶的可能结合方式。这些数据表明(E)-3的结构






























 京公网安备 11010802027423号
京公网安备 11010802027423号