当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of reactions of methoxy species with benzene and cyclohexane over H-ZSM-5 zeolites
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2015-05-05 00:00:00 , DOI: 10.1039/c5cy00600g J. N. Kondo 1, 2, 3, 4 , H. Yamazaki 1, 2, 3, 4 , T. Yokoi 1, 2, 3, 4 , T. Tatsumi 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2015-05-05 00:00:00 , DOI: 10.1039/c5cy00600g J. N. Kondo 1, 2, 3, 4 , H. Yamazaki 1, 2, 3, 4 , T. Yokoi 1, 2, 3, 4 , T. Tatsumi 1, 2, 3, 4
Affiliation
|
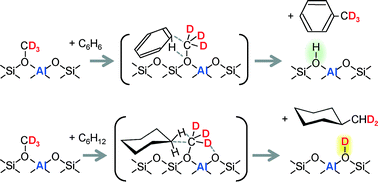
The first-step reaction mechanisms of d3-methoxy (OCD3) species on H-ZSM-5 zeolite with benzene and cyclohexane were directly observed by infrared (IR) spectroscopy. Only toluene was produced in the gas phase at the beginning, simultaneous with the consumption of methoxy species on the surface at 473 K. Acidic OH groups were formed in place of d3-methoxy groups for the reaction with benzene, while OD groups were recovered for the reaction with cyclohexane, similar to the case of light olefins. Thus, the presence of different methylation mechanisms of methoxy species is confirmed; the CD3 unit reacted with benzene as in the cases of amines and dimethyl ether, but the CD2 unit reacted with cyclohexane and light olefins, leaving one of the deuterium atoms on the zeolite. The difference of these mechanisms in activation energy is also estimated and used for further discussion on the mechanisms.
中文翻译:

H-ZSM-5分子筛上甲氧基与苯和环己烷的反应机理
通过红外光谱直接观察到H 3 -ZSM-5分子筛上的d 3-甲氧基(OCD 3)种类与苯和环己烷的第一步反应机理。开始时在气相中仅生成甲苯,同时在473 K下消耗了表面上的甲氧基类。形成了酸性OH基团,代替d 3-甲氧基与苯反应,同时回收了OD基团。与环己烷的反应,类似于轻质烯烃的情况。因此,证实了甲氧基物质存在不同的甲基化机理。在胺和二甲醚的情况下,CD 3单元与苯反应,但是CD 2单元与环己烷和轻质烯烃反应,在沸石上留下氘原子之一。还估计了这些机制在活化能方面的差异,并将其用于对该机制的进一步讨论。
更新日期:2015-05-05
中文翻译:

H-ZSM-5分子筛上甲氧基与苯和环己烷的反应机理
通过红外光谱直接观察到H 3 -ZSM-5分子筛上的d 3-甲氧基(OCD 3)种类与苯和环己烷的第一步反应机理。开始时在气相中仅生成甲苯,同时在473 K下消耗了表面上的甲氧基类。形成了酸性OH基团,代替d 3-甲氧基与苯反应,同时回收了OD基团。与环己烷的反应,类似于轻质烯烃的情况。因此,证实了甲氧基物质存在不同的甲基化机理。在胺和二甲醚的情况下,CD 3单元与苯反应,但是CD 2单元与环己烷和轻质烯烃反应,在沸石上留下氘原子之一。还估计了这些机制在活化能方面的差异,并将其用于对该机制的进一步讨论。































 京公网安备 11010802027423号
京公网安备 11010802027423号