当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomolecular Interaction Assays Identified Dual Inhibitors of Glutaminase and Glutamate Dehydrogenase That Disrupt Mitochondrial Function and Prevent Growth of Cancer Cells
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-01-26 00:00:00 , DOI: 10.1021/acs.analchem.6b03849
Min Zhu 1 , Jinzhang Fang 1 , Jingjing Zhang 1 , Zheng Zhang 1 , Jianhui Xie 1 , Yan Yu 1 , Jennifer Jin Ruan 2 , Zhao Chen 1 , Wei Hou 1 , Gensheng Yang 1 , Weike Su 1 , Benfang Helen Ruan 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-01-26 00:00:00 , DOI: 10.1021/acs.analchem.6b03849
Min Zhu 1 , Jinzhang Fang 1 , Jingjing Zhang 1 , Zheng Zhang 1 , Jianhui Xie 1 , Yan Yu 1 , Jennifer Jin Ruan 2 , Zhao Chen 1 , Wei Hou 1 , Gensheng Yang 1 , Weike Su 1 , Benfang Helen Ruan 1
Affiliation
|
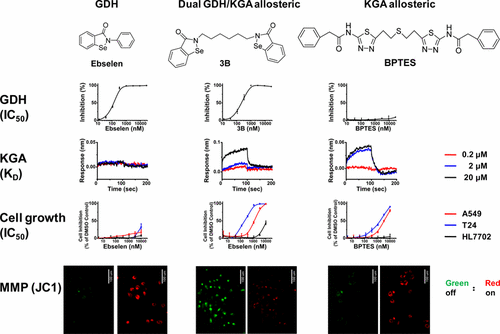
Glutaminase (KGA/isoenzyme GAC) is an emerging and important drug target for cancer. Traditional methods for assaying glutaminase activity are coupled with several other enzymes. Such coupled assays do not permit the direct and stringent characterization of specific glutaminase inhibitors. Ebselen was identified as a potent 9 nM KGA inhibitor in the KGA/glutamate oxidase (GO)/horse radish peroxidase (HRP) coupled assay but showed very weak activity in inhibiting the growth of glutamine-dependent cancer cells. For rigorous characterization, we developed a direct kinetic binding assay for KGA using bio-layer interferometry (BLI) as the detection method; Ebselen was identified as a GDH inhibitor but not a KGA inhibitor. Furthermore, we designed and synthesized several benzo[d][1,2]selenazol-3(2H)-one dimers which were subjected to SAR analysis by several glutaminolysis specific biochemical and cell based assays. Novel glutamate dehydrogenase (GDH) or dual KGA/GDH inhibitors were discovered from the synthetic compounds; the dual inhibitors completely disrupt mitochondrial function and demonstrate potent anticancer activity with a minimum level of toxicity.
中文翻译:

生物分子相互作用测定法鉴定了谷氨酰胺酶和谷氨酸脱氢酶的双重抑制剂,破坏了线粒体功能并阻止了癌细胞的生长
谷氨酰胺酶(KGA /同工酶GAC)是一种新兴的重要癌症靶标。用于测定谷氨酰胺酶活性的传统方法与几种其他酶偶联。这种偶联测定不允许直接和严格表征特异性谷氨酰胺酶抑制剂。Ebselen在KGA /谷氨酸氧化酶(GO)/辣根过氧化物酶(HRP)耦合分析中被鉴定为有效的9 nM KGA抑制剂,但在抑制谷氨酰胺依赖性癌细胞生长方面表现出非常弱的活性。为了进行严格的表征,我们使用生物层干涉法(BLI)作为检测方法开发了KGA的直接动力学结合测定法;Ebselen被鉴定为GDH抑制剂,但不是KGA抑制剂。此外,我们设计并合成了几种苯并[ d]。] [1,2] selenazol-3(2H)-one二聚体,通过几种谷氨酰胺分解特异性生化和基于细胞的分析进行了SAR分析。从合成化合物中发现了新型谷氨酸脱氢酶(GDH)或双重KGA / GDH抑制剂。双重抑制剂可完全破坏线粒体功能,并显示出强大的抗癌活性,且毒性最低。
更新日期:2017-01-26
中文翻译:

生物分子相互作用测定法鉴定了谷氨酰胺酶和谷氨酸脱氢酶的双重抑制剂,破坏了线粒体功能并阻止了癌细胞的生长
谷氨酰胺酶(KGA /同工酶GAC)是一种新兴的重要癌症靶标。用于测定谷氨酰胺酶活性的传统方法与几种其他酶偶联。这种偶联测定不允许直接和严格表征特异性谷氨酰胺酶抑制剂。Ebselen在KGA /谷氨酸氧化酶(GO)/辣根过氧化物酶(HRP)耦合分析中被鉴定为有效的9 nM KGA抑制剂,但在抑制谷氨酰胺依赖性癌细胞生长方面表现出非常弱的活性。为了进行严格的表征,我们使用生物层干涉法(BLI)作为检测方法开发了KGA的直接动力学结合测定法;Ebselen被鉴定为GDH抑制剂,但不是KGA抑制剂。此外,我们设计并合成了几种苯并[ d]。] [1,2] selenazol-3(2H)-one二聚体,通过几种谷氨酰胺分解特异性生化和基于细胞的分析进行了SAR分析。从合成化合物中发现了新型谷氨酸脱氢酶(GDH)或双重KGA / GDH抑制剂。双重抑制剂可完全破坏线粒体功能,并显示出强大的抗癌活性,且毒性最低。

































 京公网安备 11010802027423号
京公网安备 11010802027423号