当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Synthesis of Ammonia at Room Temperature and Atmospheric Pressure from Water and Nitrogen on a Carbon‐Nanotube‐Based Electrocatalyst
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-01-27 , DOI: 10.1002/anie.201609533
Shiming Chen 1 , Siglinda Perathoner 1 , Claudio Ampelli 1 , Chalachew Mebrahtu 1 , Dangsheng Su 2 , Gabriele Centi 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-01-27 , DOI: 10.1002/anie.201609533
Shiming Chen 1 , Siglinda Perathoner 1 , Claudio Ampelli 1 , Chalachew Mebrahtu 1 , Dangsheng Su 2 , Gabriele Centi 1
Affiliation
|
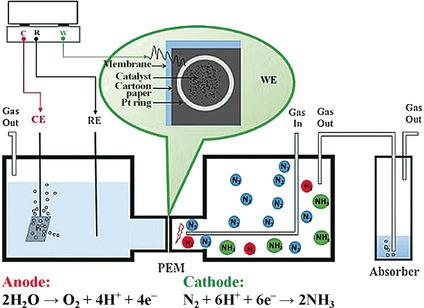
Ammonia is synthesized directly from water and N2 at room temperature and atmospheric pressure in a flow electrochemical cell operating in gas phase (half‐cell for the NH3 synthesis). Iron supported on carbon nanotubes (CNTs) was used as the electrocatalyst in this half‐cell. A rate of ammonia formation of 2.2×10−3 g m−2 h−1 was obtained at room temperature and atmospheric pressure in a flow of N2, with stable behavior for at least 60 h of reaction, under an applied potential of −2.0 V. This value is higher than the rate of ammonia formation obtained using noble metals (Ru/C) under comparable reaction conditions. Furthermore, hydrogen gas with a total Faraday efficiency as high as 95.1 % was obtained. Data also indicate that the active sites in NH3 electrocatalytic synthesis may be associated to specific carbon sites formed at the interface between iron particles and CNT and able to activate N2, making it more reactive towards hydrogenation.
m−2 h−1 was obtained at room temperature and atmospheric pressure in a flow of N2, with stable behavior for at least 60 h of reaction, under an applied potential of −2.0 V. This value is higher than the rate of ammonia formation obtained using noble metals (Ru/C) under comparable reaction conditions. Furthermore, hydrogen gas with a total Faraday efficiency as high as 95.1 % was obtained. Data also indicate that the active sites in NH3 electrocatalytic synthesis may be associated to specific carbon sites formed at the interface between iron particles and CNT and able to activate N2, making it more reactive towards hydrogenation.
中文翻译:

碳纳米管基电催化剂上水和氮在室温和大气压下电催化合成氨
氨是在室温和大气压下在气相中运行的流通式电化学池(用于合成NH 3的半池)中直接由水和N 2合成的。在该半电池中,负载在碳纳米管(CNTs)上的铁用作电催化剂。在室温和大气压下,在N 2流中,氨形成速率为2.2×10 -3 g m -2 h -1 ,在-2.0 V的施加电势下,对于至少60 h的反应具有稳定的行为。该值高于在可比的反应条件下使用贵金属(Ru / C)获得的氨形成速率。此外,获得了法拉第总效率高达95.1%的氢气。数据还表明,NH 3电催化合成中的活性位点可能与在铁颗粒和CNT之间的界面处形成的特定碳位点相关,并且能够活化N 2,使其对氢化反应性更高。
,在-2.0 V的施加电势下,对于至少60 h的反应具有稳定的行为。该值高于在可比的反应条件下使用贵金属(Ru / C)获得的氨形成速率。此外,获得了法拉第总效率高达95.1%的氢气。数据还表明,NH 3电催化合成中的活性位点可能与在铁颗粒和CNT之间的界面处形成的特定碳位点相关,并且能够活化N 2,使其对氢化反应性更高。
更新日期:2017-01-27
 m−2 h−1 was obtained at room temperature and atmospheric pressure in a flow of N2, with stable behavior for at least 60 h of reaction, under an applied potential of −2.0 V. This value is higher than the rate of ammonia formation obtained using noble metals (Ru/C) under comparable reaction conditions. Furthermore, hydrogen gas with a total Faraday efficiency as high as 95.1 % was obtained. Data also indicate that the active sites in NH3 electrocatalytic synthesis may be associated to specific carbon sites formed at the interface between iron particles and CNT and able to activate N2, making it more reactive towards hydrogenation.
m−2 h−1 was obtained at room temperature and atmospheric pressure in a flow of N2, with stable behavior for at least 60 h of reaction, under an applied potential of −2.0 V. This value is higher than the rate of ammonia formation obtained using noble metals (Ru/C) under comparable reaction conditions. Furthermore, hydrogen gas with a total Faraday efficiency as high as 95.1 % was obtained. Data also indicate that the active sites in NH3 electrocatalytic synthesis may be associated to specific carbon sites formed at the interface between iron particles and CNT and able to activate N2, making it more reactive towards hydrogenation.
中文翻译:

碳纳米管基电催化剂上水和氮在室温和大气压下电催化合成氨
氨是在室温和大气压下在气相中运行的流通式电化学池(用于合成NH 3的半池)中直接由水和N 2合成的。在该半电池中,负载在碳纳米管(CNTs)上的铁用作电催化剂。在室温和大气压下,在N 2流中,氨形成速率为2.2×10 -3 g m -2 h -1
 ,在-2.0 V的施加电势下,对于至少60 h的反应具有稳定的行为。该值高于在可比的反应条件下使用贵金属(Ru / C)获得的氨形成速率。此外,获得了法拉第总效率高达95.1%的氢气。数据还表明,NH 3电催化合成中的活性位点可能与在铁颗粒和CNT之间的界面处形成的特定碳位点相关,并且能够活化N 2,使其对氢化反应性更高。
,在-2.0 V的施加电势下,对于至少60 h的反应具有稳定的行为。该值高于在可比的反应条件下使用贵金属(Ru / C)获得的氨形成速率。此外,获得了法拉第总效率高达95.1%的氢气。数据还表明,NH 3电催化合成中的活性位点可能与在铁颗粒和CNT之间的界面处形成的特定碳位点相关,并且能够活化N 2,使其对氢化反应性更高。

































 京公网安备 11010802027423号
京公网安备 11010802027423号