当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aminocarbonylation (hydrazinocarbonylation) of iodoalkenes and iodoarenes
Tetrahedron ( IF 2.1 ) Pub Date : 2017-01-23 23:35:23 Máté Gergely, László Kollár
Tetrahedron ( IF 2.1 ) Pub Date : 2017-01-23 23:35:23 Máté Gergely, László Kollár
|
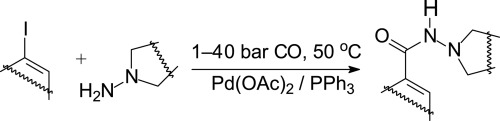
Iodoalkenes such as 1-iodocyclohexene, 4-tert-butyl-1-iodocyclohexene, α-iodostyrene and 17-iodoandrost-16-ene were aminocarbonylated in palladium-catalysed reaction using 1,1-disubstituted (cyclic) hydrazines (3-amino-3-azabicyclo[3.3.0]octane and (S)-1-amino-2-methoxymethylpyrrolidine (SAMP)/(R)-1-amino-2-methoxymethyl-pyrrolidine (RAMP)) as N-nucleophiles. The corresponding hydrazides were formed in moderate to high yields. The hydrazinocarbonylation of iodobenzene using the above 1,1-disubstituted hydrazines resulted in a rather complex reaction mixture due to two major types of side-reactions: i) the deamination of the 3-amino-3-azabicyclo[3.3.0]octane, and ii) the double carbon monoxide insertion. In this way, in addition to the expected benzoylhydrazide derivative, phenylglyoxylhydrazide (double CO insertion product) and benzamide (‘deamination’ product) were also formed. By the appropriate modification of the reaction conditions, good selectivities towards the target compounds were achieved even in these cases. The formation of the products/side-products were rationalized on the basis of a simplified catalytic cycle.
中文翻译:

碘代烯烃和碘代芳烃的氨基羰基化(肼基羰基化)
在钯催化的反应中,使用1,1-二取代(环状)肼(3-氨基- 3-氮杂双环[3.3.0]辛烷和(S)-1-氨基-2-甲氧基甲基吡咯烷(SAMP)/(R)-1-氨基-2-甲氧基甲基吡咯烷(RAMP))作为N-亲核试剂。相应的酰肼以中等至高产率形成。由于上述两种主要的副反应类型,使用上述1,1-二取代肼进行碘苯的肼基羰基化反应时,反应混合物相当复杂:i)3-氨基-3-氮杂双环[3.3.0]辛烷的脱氨基; ii)一氧化碳的双重插入。这样,除了预期的苯甲酰肼衍生物之外,还形成了苯乙氧基酰肼(双CO插入产物)和苯甲酰胺(“脱氨”产物)。通过适当改变反应条件,即使在这些情况下,也可以实现对目标化合物的良好选择性。在简化的催化循环的基础上,合理化了产物/副产物的形成。
更新日期:2017-01-24
中文翻译:

碘代烯烃和碘代芳烃的氨基羰基化(肼基羰基化)
在钯催化的反应中,使用1,1-二取代(环状)肼(3-氨基- 3-氮杂双环[3.3.0]辛烷和(S)-1-氨基-2-甲氧基甲基吡咯烷(SAMP)/(R)-1-氨基-2-甲氧基甲基吡咯烷(RAMP))作为N-亲核试剂。相应的酰肼以中等至高产率形成。由于上述两种主要的副反应类型,使用上述1,1-二取代肼进行碘苯的肼基羰基化反应时,反应混合物相当复杂:i)3-氨基-3-氮杂双环[3.3.0]辛烷的脱氨基; ii)一氧化碳的双重插入。这样,除了预期的苯甲酰肼衍生物之外,还形成了苯乙氧基酰肼(双CO插入产物)和苯甲酰胺(“脱氨”产物)。通过适当改变反应条件,即使在这些情况下,也可以实现对目标化合物的良好选择性。在简化的催化循环的基础上,合理化了产物/副产物的形成。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号