当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidation of Chloride and Subsequent Chlorination of Organic Compounds by Oxoiron(IV) Porphyrin π‐Cation Radicals
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2011-09-12 , DOI: 10.1002/anie.201104461 Zhiqi Cong , Takuya Kurahashi , Hiroshi Fujii
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2011-09-12 , DOI: 10.1002/anie.201104461 Zhiqi Cong , Takuya Kurahashi , Hiroshi Fujii
|
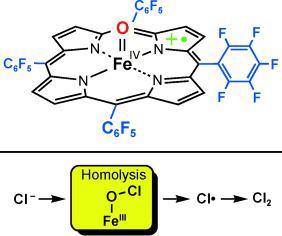
Ironing it out: Oxoiron(IV) porphyrin π‐cation radical complexes (see figure) serve as models for the oxidation of Cl− into an active chlorinating reagent that chlorinates various organic compounds. Evidence suggests that Cl− is oxidized to Cl2 via Cl.. The mechanism involving either direct electron transfer or iron(III) hypochlorite formation, and then homolysis of the ClO bond is discussed.

中文翻译:

氧化铁(IV)卟啉π-阳离子自由基对氯化物的氧化和随后对有机化合物的氯化
熨烫出来:Oxoiron(IV)卟啉π-阳离子自由基复合物(见图)作为模型Cl组成的氧化-成活性氯化试剂chlorinates各种有机化合物。有证据表明,氯-被氧化成氯2地通过Cl 。。涉及的Cl的直接电子转移或铁(III)的次氯酸盐的形成,然后均裂机理 O键进行了讨论。

更新日期:2011-09-12

中文翻译:

氧化铁(IV)卟啉π-阳离子自由基对氯化物的氧化和随后对有机化合物的氯化
熨烫出来:Oxoiron(IV)卟啉π-阳离子自由基复合物(见图)作为模型Cl组成的氧化-成活性氯化试剂chlorinates各种有机化合物。有证据表明,氯-被氧化成氯2地通过Cl 。。涉及的Cl的直接电子转移或铁(III)的次氯酸盐的形成,然后均裂机理 O键进行了讨论。



















































 京公网安备 11010802027423号
京公网安备 11010802027423号