当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereospecific Intramolecular Arylation of 2- and 3-Pyridyl Substituted Alkylamines via Configurationally Stable α-Pyridyl Organolithiums
Organic Letters ( IF 4.9 ) Pub Date : 2017-01-18 00:00:00 , DOI: 10.1021/acs.orglett.6b03603 Julien Maury 1, 2 , Wojciech Zawodny 1 , Jonathan Clayden 2
Organic Letters ( IF 4.9 ) Pub Date : 2017-01-18 00:00:00 , DOI: 10.1021/acs.orglett.6b03603 Julien Maury 1, 2 , Wojciech Zawodny 1 , Jonathan Clayden 2
Affiliation
|
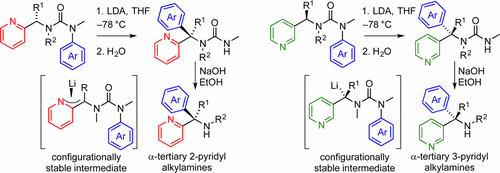
Treatment of N′-aryl urea derivatives of enantiomerically enriched α-(2-pyridyl) and α-(3-pyridyl)alkylamines with a base leads to the migration of the N′-aryl substituent from N to C in a ‘nonclassical’ intramolecular nucleophilic aromatic substitution reaction. Both electron-rich and -poor rings migrate successfully. A new quaternary stereogenic center is formed adjacent to the pyridine ring with high stereospecificity, even when the intermediate anion is a presumably planar 2-picolyllithium. Base hydrolysis of the urea gives enantiomerically enriched α-pyridylalkylamines.
中文翻译:

通过构型稳定的α-吡啶基有机锂的2-和3-吡啶基取代的烷基胺的立体特异性分子内芳基化
用碱处理对映体富集的α-(2-吡啶基)和α-(3-吡啶基)烷基胺的N'-芳基脲衍生物会导致N'-芳基取代基从N迁移至C的“非经典”状态分子内亲核芳族取代反应。富电子环和贫电子环均成功迁移。即使当中间阴离子可能是平面的2-甲基吡啶基锂时,在吡啶环附近也会形成一个新的立体立体中心,该中心具有很高的立体特异性。脲的碱水解得到对映体富集的α-吡啶基烷基胺。
更新日期:2017-01-18
中文翻译:

通过构型稳定的α-吡啶基有机锂的2-和3-吡啶基取代的烷基胺的立体特异性分子内芳基化
用碱处理对映体富集的α-(2-吡啶基)和α-(3-吡啶基)烷基胺的N'-芳基脲衍生物会导致N'-芳基取代基从N迁移至C的“非经典”状态分子内亲核芳族取代反应。富电子环和贫电子环均成功迁移。即使当中间阴离子可能是平面的2-甲基吡啶基锂时,在吡啶环附近也会形成一个新的立体立体中心,该中心具有很高的立体特异性。脲的碱水解得到对映体富集的α-吡啶基烷基胺。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号