当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilizing Au(III) in supported-ionic-liquid-phase (SILP) catalyst using CuCl2 via a redox mechanism
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-01-19 20:52:40
Jia Zhao, Yi Yu, Xiaolong Xu, Shuxia Di, Bolin Wang, Hao Xu, Jun Ni, LingLing Guo, Zhiyan Pan, Xiaonian Li
Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2017-01-19 20:52:40
Jia Zhao, Yi Yu, Xiaolong Xu, Shuxia Di, Bolin Wang, Hao Xu, Jun Ni, LingLing Guo, Zhiyan Pan, Xiaonian Li
|
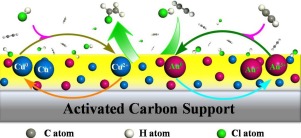
High-valent Au(III) complexes have attracted much attention as catalysts in many reactions. Nevertheless Au(III) catalysts suffer from instability of the oxidized metal complexes during preparation and use. Herein, we demonstrated that Au(III) catalysts can be stabilized against reduction to metallic Au0 by modifying supported-ionic-liquid-phase-stabilized Au(III) catalyst with CuCl2. It was found that the that reduced Au0 could be re-oxidized in situ to Au3+ species by CuCl2 during the reaction and further stabilized by the electron transfer from Cu to these active species. When evaluated in the acetylene hydrochlorination reaction, the Au-Cu-IL/AC catalyst displayed an excellent specific activity with the turnover frequency (TOF) as high as 168.5h−1 and more than 99.8% selectivity for the product, vinyl chloride (VCM). Furthermore, the Au-Cu-IL/AC catalyst demonstrated a stable catalytic performance with a negligible loss of C2H2 conversion after 500h under typical industrial reaction conditions for acetylene hydrochlorination. Therefore, the findings of this work provide an efficient approach for designing stable high-valent metals for long-term operation, and also pave the way for the application of Au-Cu-IL/AC catalyst in industrial VCM production.
中文翻译:

通过氧化还原机理使用CuCl2稳定负载型离子液体(SILP)催化剂中的Au(III)
在许多反应中,高价Au(III)络合物作为催化剂已引起广泛关注。然而,Au(III)催化剂在制备和使用过程中遭受氧化金属配合物的不稳定性。在本文中,我们证明了可以通过用CuCl 2改性负载型离子液体稳定的Au(III)催化剂来稳定Au(III)催化剂,以防止还原为金属Au 0。发现还原的Au 0可以被CuCl 2原位氧化为Au 3+。在反应过程中,并通过电子从铜转移到这些活性物质而进一步稳定。当在乙炔氢氯化反应中进行评估时,Au-Cu-IL / AC催化剂显示出优异的比活性,其周转频率(TOF)高达168.5h -1,并且对产物氯乙烯(VCM)的选择性超过99.8%。 )。此外,Au-Cu-IL / AC催化剂显示出稳定的催化性能,而C 2 H 2的损失可忽略不计在典型的工业反应条件下,经过500小时的乙炔盐酸盐转化反应。因此,这项工作的发现为设计长期运行的稳定高价金属提供了一种有效的方法,也为在工业VCM生产中应用Au-Cu-IL / AC催化剂铺平了道路。
更新日期:2017-01-20
中文翻译:

通过氧化还原机理使用CuCl2稳定负载型离子液体(SILP)催化剂中的Au(III)
在许多反应中,高价Au(III)络合物作为催化剂已引起广泛关注。然而,Au(III)催化剂在制备和使用过程中遭受氧化金属配合物的不稳定性。在本文中,我们证明了可以通过用CuCl 2改性负载型离子液体稳定的Au(III)催化剂来稳定Au(III)催化剂,以防止还原为金属Au 0。发现还原的Au 0可以被CuCl 2原位氧化为Au 3+。在反应过程中,并通过电子从铜转移到这些活性物质而进一步稳定。当在乙炔氢氯化反应中进行评估时,Au-Cu-IL / AC催化剂显示出优异的比活性,其周转频率(TOF)高达168.5h -1,并且对产物氯乙烯(VCM)的选择性超过99.8%。 )。此外,Au-Cu-IL / AC催化剂显示出稳定的催化性能,而C 2 H 2的损失可忽略不计在典型的工业反应条件下,经过500小时的乙炔盐酸盐转化反应。因此,这项工作的发现为设计长期运行的稳定高价金属提供了一种有效的方法,也为在工业VCM生产中应用Au-Cu-IL / AC催化剂铺平了道路。







































 京公网安备 11010802027423号
京公网安备 11010802027423号