当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of (R)‐2‐Chloro‐1‐(2,4‐dichlorophenyl)ethanol with a Ketoreductase from Scheffersomyces stipitis CBS 6045
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-01-18 , DOI: 10.1002/adsc.201601003 Yue-Peng Shang 1 , Qi Chen 1 , Xu-Dong Kong 1 , Yu-Jun Zhang 1 , Jian-He Xu 1 , Hui-Lei Yu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-01-18 , DOI: 10.1002/adsc.201601003 Yue-Peng Shang 1 , Qi Chen 1 , Xu-Dong Kong 1 , Yu-Jun Zhang 1 , Jian-He Xu 1 , Hui-Lei Yu 1
Affiliation
|
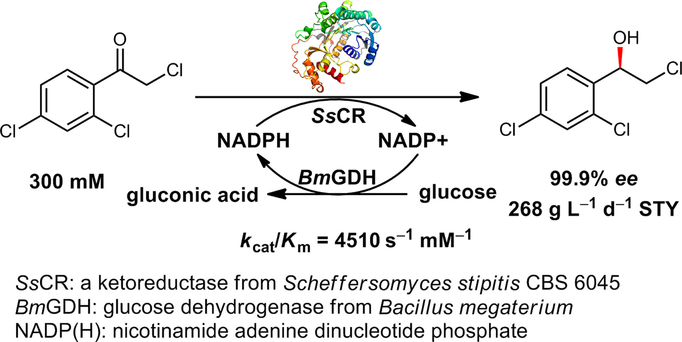
By enzyme screening, a ketoreductase cloned from Scheffersomyces stipitis CBS 6045 and named SsCR was identified that could catalyze the asymmetric hydrogenation of a variety of aromatic ketones. SsCR exhibited a specific activity of 65 U mg−1 protein and excellent enantioselectivity (99.9% ee) towards the hydrophobic substrate 2‐chloro‐1‐(2,4‐dichlorophenyl)ethanone, which is an intermediate in the synthesis of common antifungal agents such as miconazole, econazole and sertaconazole. The kinetic parameter kcat/Km was 4.51×103 s−1 mM−1, showing the great catalytic efficiency of SsCR towards this substrate. Molecular dynamic simulation results shed light on the higher substrate binding free energy change for this substrate relative to other substrates. Based on the good catalytic properties of SsCR, (R)‐2‐chloro‐1‐(2,4‐dichlorophenyl)ethanol could be obtained with a space‐time yield (STY) of up to 268 g L−1 d−1 without any additional cofactor required in the reductive reaction process. On scaling up the bioreaction, the (R)‐alcohol was isolated with 88.2% yield and 99.9% ee. The environmental factor (E factor) of this reaction was 7.25 when process water was excluded.
中文翻译:

钝角拟南芥CBS 6045中的酮还原酶高效合成(R)-2-氯-1-(2,4-二氯苯基)乙醇
通过酶筛选,鉴定了从钝叶拟南芥CBS 6045中克隆的酮还原酶,命名为Ss CR,它可以催化多种芳族酮的不对称氢化。Ss CR表现出65 U mg -1蛋白的比活和对疏水底物2-氯-1-(2,4-二氯苯基)乙酮的出色对映选择性(99.9%ee),后者是常见抗真菌药物合成的中间体咪康唑,益康唑和舍他康唑等药物。动力学参数k cat / K m为4.51×10 3 s -1 mM -1,显示出Ss CR对这种底物的巨大催化效率。分子动力学模拟结果揭示了该底物相对于其他底物的更高的底物结合自由能变化。基于良好的催化性能SS CR,(- [R)-2-氯-1-(2,4-二氯苯基)乙醇可以与最多268克L-的时空产率(STY)获得-1 d -在还原反应过程中不需要任何额外的辅助因子的图1所示的方法。在扩大生物反应时,分离出(R)醇的产率为88.2%,ee为99.9%。当排除工艺用水时,该反应的环境因子(E因子)为7.25。
更新日期:2017-01-18
中文翻译:

钝角拟南芥CBS 6045中的酮还原酶高效合成(R)-2-氯-1-(2,4-二氯苯基)乙醇
通过酶筛选,鉴定了从钝叶拟南芥CBS 6045中克隆的酮还原酶,命名为Ss CR,它可以催化多种芳族酮的不对称氢化。Ss CR表现出65 U mg -1蛋白的比活和对疏水底物2-氯-1-(2,4-二氯苯基)乙酮的出色对映选择性(99.9%ee),后者是常见抗真菌药物合成的中间体咪康唑,益康唑和舍他康唑等药物。动力学参数k cat / K m为4.51×10 3 s -1 mM -1,显示出Ss CR对这种底物的巨大催化效率。分子动力学模拟结果揭示了该底物相对于其他底物的更高的底物结合自由能变化。基于良好的催化性能SS CR,(- [R)-2-氯-1-(2,4-二氯苯基)乙醇可以与最多268克L-的时空产率(STY)获得-1 d -在还原反应过程中不需要任何额外的辅助因子的图1所示的方法。在扩大生物反应时,分离出(R)醇的产率为88.2%,ee为99.9%。当排除工艺用水时,该反应的环境因子(E因子)为7.25。

































 京公网安备 11010802027423号
京公网安备 11010802027423号