当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solving the Puzzle of One‐Carbon Loss in Ripostatin Biosynthesis
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-01-18 , DOI: 10.1002/anie.201609950 Chengzhang Fu 1 , David Auerbach 1 , Yanyan Li 1, 2 , Ullrich Scheid 1, 3 , Eva Luxenburger 1, 3 , Ronald Garcia 1, 3 , Herbert Irschik 4 , Rolf Müller 1, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-01-18 , DOI: 10.1002/anie.201609950 Chengzhang Fu 1 , David Auerbach 1 , Yanyan Li 1, 2 , Ullrich Scheid 1, 3 , Eva Luxenburger 1, 3 , Ronald Garcia 1, 3 , Herbert Irschik 4 , Rolf Müller 1, 3
Affiliation
|
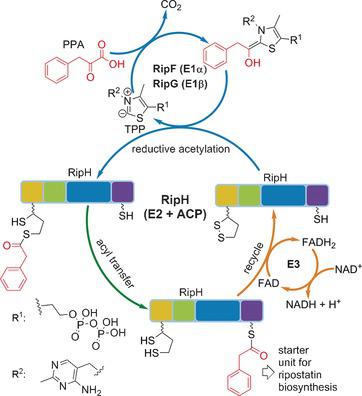
Ripostatin is a promising antibiotic that inhibits RNA polymerase by binding to a novel binding site. In this study, the characterization of the biosynthetic gene cluster of ripostatin, which is a peculiar polyketide synthase (PKS) hybrid cluster encoding cis‐ and trans‐acyltransferase PKS genes, is reported. Moreover, an unprecedented mechanism for phenyl acetic acid formation and loading as a starter unit was discovered. This phenyl‐C2 unit is derived from phenylpyruvate (phenyl‐C3) and the mechanism described herein explains the mysterious loss of one carbon atom in ripostatin biosynthesis from the phenyl‐C3 precursor. Through in vitro reconstitution of the whole loading process, a pyruvate dehydrogenase like protein complex was revealed that performs thiamine pyrophosphate dependent decarboxylation of phenylpyruvate to form a phenylacetyl‐S‐acyl carrier protein species, which is supplied to the subsequent biosynthetic assembly line for chain extension to finally yield ripostatin.
中文翻译:

解决Ripostatin生物合成中单碳损失的难题
Ripostatin是一种有前途的抗生素,可通过与新的结合位点结合抑制RNA聚合酶。在这项研究中,已报道了ripostatin的生物合成基因簇的特征,这是一种独特的聚酮化合物合酶(PKS)杂种簇,编码顺式和反式酰基转移酶PKS基因。此外,发现了空前的苯乙酸形成和装载作为起始单元的机理。该苯基C2单元衍生自丙酮酸苯基酯(苯基C3),本文所述的机理解释了从苯基C3前体进行的利托伐他汀生物合成中一个碳原子的神秘损失。通过整个装载过程的体外重组,发现丙酮酸脱氢酶样蛋白质复合物可进行硫胺素焦磷酸依赖的苯丙酮酸脱羧反应,形成苯乙酰基-S酰基载体蛋白种类,将其提供给随后的生物合成装配线以进行链延伸,从而最终产生ripostatin。
更新日期:2017-01-18
中文翻译:

解决Ripostatin生物合成中单碳损失的难题
Ripostatin是一种有前途的抗生素,可通过与新的结合位点结合抑制RNA聚合酶。在这项研究中,已报道了ripostatin的生物合成基因簇的特征,这是一种独特的聚酮化合物合酶(PKS)杂种簇,编码顺式和反式酰基转移酶PKS基因。此外,发现了空前的苯乙酸形成和装载作为起始单元的机理。该苯基C2单元衍生自丙酮酸苯基酯(苯基C3),本文所述的机理解释了从苯基C3前体进行的利托伐他汀生物合成中一个碳原子的神秘损失。通过整个装载过程的体外重组,发现丙酮酸脱氢酶样蛋白质复合物可进行硫胺素焦磷酸依赖的苯丙酮酸脱羧反应,形成苯乙酰基-S酰基载体蛋白种类,将其提供给随后的生物合成装配线以进行链延伸,从而最终产生ripostatin。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号