当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structurally and Stereochemically Diverse Tetrahydropyran Synthesis through Oxidative C?H Bond Activation
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2010-03-22 , DOI: 10.1002/anie.201000033 Lei Liu 1 , Paul E Floreancig
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2010-03-22 , DOI: 10.1002/anie.201000033 Lei Liu 1 , Paul E Floreancig
Affiliation
|
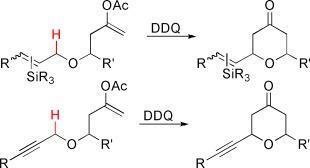
Simple as pyran: Vinylsilane‐substituted tetrahydropyrans, readily accessed through the oxidative CH bond functionalization of silylallylic or propargylic ethers (see scheme; DDQ=2,3‐dichloro‐5,6‐dicyano‐1,4‐benzoquinone), are versatile substrates for a wide range of functional group interconversions and stereocontrolled additions. These processes have applications in convergent target‐ or diversity‐oriented synthesis strategies.

中文翻译:

通过氧化 C?H 键活化合成结构和立体化学多样化的四氢吡喃
像吡喃一样简单:乙烯基硅烷取代的四氢吡喃,可以通过甲硅烷基烯丙基醚或炔丙基醚的氧化C - H键官能化轻松获得(参见方案;DDQ=2,3-二氯-5,6-二氰基-1,4-苯醌),是用于各种官能团相互转化和立体控制加成的多功能底物。这些过程可应用于聚合目标或多样性导向的合成策略。

更新日期:2010-03-22

中文翻译:

通过氧化 C?H 键活化合成结构和立体化学多样化的四氢吡喃
像吡喃一样简单:乙烯基硅烷取代的四氢吡喃,可以通过甲硅烷基烯丙基醚或炔丙基醚的氧化C - H键官能化轻松获得(参见方案;DDQ=2,3-二氯-5,6-二氰基-1,4-苯醌),是用于各种官能团相互转化和立体控制加成的多功能底物。这些过程可应用于聚合目标或多样性导向的合成策略。
































 京公网安备 11010802027423号
京公网安备 11010802027423号