Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electroreduction of Viologen Phenyl Diazonium Salts as a Strategy To Control Viologen Coverage on Electrodes
Langmuir ( IF 3.7 ) Pub Date : 2017-01-13 00:00:00 , DOI: 10.1021/acs.langmuir.6b04317 Liangcheng Cao 1 , Gan Fang 1 , Yuechuan Wang 2
Langmuir ( IF 3.7 ) Pub Date : 2017-01-13 00:00:00 , DOI: 10.1021/acs.langmuir.6b04317 Liangcheng Cao 1 , Gan Fang 1 , Yuechuan Wang 2
Affiliation
|
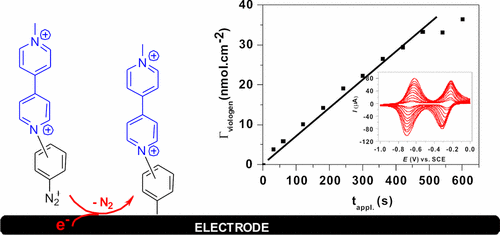
A majority of the reported electrografting of aryldiazonium salts result in the formation of covalently attached films with a limited surface coverage of below 5 nmol·cm–2. Herein, we report the preparation of higher-thickness redox-active viologen-grafted electrodes from the electroreduction of viologen phenyl diazonium salts, by either cyclic voltammetric (CV) sweeps or electrolysis using a fixed potential. Both of the methodologies were successfully applied for various conductive surfaces, including glassy carbon (GC), gold disc, indium tin oxide glass, mesoporous TiO2 electrodes, and 3D compacted carbon fibers. A robust maximal viologen coverage, Γviologen = 9.5 nmol·cm–2, was achieved on a GC electrode by CV electroreduction. Electroreduction held at a fixed potential at Eappl. = −0.3 V can fabricate viologen-grafted electrodes with Γviologen in the range of 0–37 nmol·cm–2 in a controllable way, by simply adjusting the electrodeposition time tappl.. Time-dependent Γviologen were found to be 10 nmol·cm–2@2 min, 20 nmol·cm–2@4.2 min, and 30 nmol·cm–2@7 min. Furthermore, a TiO2 electrode coupled with Γviologen of 140 nmol·cm–2 exhibited electrochromic performance, with the color changing from pale yellow to blue and red brown.
中文翻译:

电还原紫精苯基重氮盐作为控制电极上紫精覆盖率的策略
多数报道的芳基重氮盐的电接枝导致形成共价结合的膜,其表面覆盖率低于5 nmol·cm –2。在本文中,我们报告了通过循环伏安法(CV)扫描或使用固定电势进行电解,从对紫精苯基重氮盐的电还原中制备更高厚度的氧化还原活性紫精接枝电极。两种方法均成功应用于各种导电表面,包括玻璃碳(GC),金盘,氧化铟锡玻璃,介孔TiO 2电极和3D压实碳纤维。稳健的最大紫精覆盖率,Γ紫精= 9.5 nmol·cm –2通过CV电解还原在GC电极上获得δ。电还原在E appl处保持固定电位。= -0.3V时可以制造具有Γ紫精接枝电极紫精·厘米0-37纳摩尔的范围-2以可控的方式,通过简单地调整电沉积时间吨申请。。发现随时间变化的Γ紫精为10 nmol·cm –2 @ 2分钟,20 nmol·cm –2 @ 4.2分钟和30 nmol·cm –2 @ 7分钟。此外,TiO 2电极结合了140 nmol·cm –2的Γ紫精 表现出电致变色性能,颜色从浅黄色变为蓝色和红棕色。
更新日期:2017-01-13
中文翻译:

电还原紫精苯基重氮盐作为控制电极上紫精覆盖率的策略
多数报道的芳基重氮盐的电接枝导致形成共价结合的膜,其表面覆盖率低于5 nmol·cm –2。在本文中,我们报告了通过循环伏安法(CV)扫描或使用固定电势进行电解,从对紫精苯基重氮盐的电还原中制备更高厚度的氧化还原活性紫精接枝电极。两种方法均成功应用于各种导电表面,包括玻璃碳(GC),金盘,氧化铟锡玻璃,介孔TiO 2电极和3D压实碳纤维。稳健的最大紫精覆盖率,Γ紫精= 9.5 nmol·cm –2通过CV电解还原在GC电极上获得δ。电还原在E appl处保持固定电位。= -0.3V时可以制造具有Γ紫精接枝电极紫精·厘米0-37纳摩尔的范围-2以可控的方式,通过简单地调整电沉积时间吨申请。。发现随时间变化的Γ紫精为10 nmol·cm –2 @ 2分钟,20 nmol·cm –2 @ 4.2分钟和30 nmol·cm –2 @ 7分钟。此外,TiO 2电极结合了140 nmol·cm –2的Γ紫精 表现出电致变色性能,颜色从浅黄色变为蓝色和红棕色。















































 京公网安备 11010802027423号
京公网安备 11010802027423号