当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Heterocyclic (4-Phenylpiperazin-1-yl)methanones Derived from Phenoxazine and Phenothiazine as Highly Potent Inhibitors of Tubulin Polymerization
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-01-13 00:00:00 , DOI: 10.1021/acs.jmedchem.6b01591 Helge Prinz 1 , Ann-Kathrin Ridder 1 , Kirsten Vogel 1 , Konrad J. Böhm 2 , Igor Ivanov 3 , Jahan B. Ghasemi 4 , Elham Aghaee 4 , Klaus Müller 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-01-13 00:00:00 , DOI: 10.1021/acs.jmedchem.6b01591 Helge Prinz 1 , Ann-Kathrin Ridder 1 , Kirsten Vogel 1 , Konrad J. Böhm 2 , Igor Ivanov 3 , Jahan B. Ghasemi 4 , Elham Aghaee 4 , Klaus Müller 1
Affiliation
|
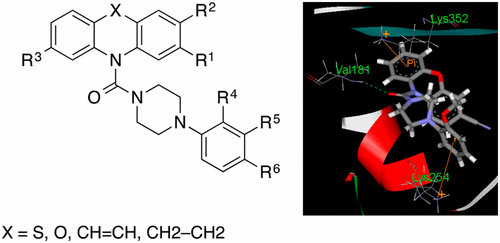
We report here a series of 27 10-(4-phenylpiperazin-1-yl)methanones derived from tricyclic heterocycles which were screened for effects on tumor cell growth, inhibition of tubulin polymerization, and induction of cell cycle arrest. Several analogues, among them the 10-(4-(3-methoxyphenyl)piperazine-1-carbonyl)-10H-phenoxazine-3-carbonitrile (16o), showed excellent antiproliferative properties, with low nanomolar GI50 values (16o, mean GI50 of 3.3 nM) against a large number (93) of cancer cell lines. Fifteen compounds potently inhibited tubulin polymerization. Analysis of cell cycle by flow cytometry revealed that inhibition of tumor cell growth was related to an induction of G2/M phase cell cycle blockade. Western blotting and molecular docking studies suggested that these compounds bind efficiently to β-tubulin at the colchicine binding site. Our studies demonstrate the suitability of the phenoxazine and phenothiazine core and also of the phenylpiperazine moiety for the development of novel and potent tubulin polymerization inhibitors.
中文翻译:

衍生自吩恶嗪和吩噻嗪的N-杂环(4-苯基哌嗪-1-基)亚甲基酮作为微管蛋白聚合的高效抑制剂
我们在这里报告了一系列从三环杂环衍生的27 10-(4-苯基哌嗪-1-基)甲亚胺,这些环经筛选对肿瘤细胞生长,微管蛋白聚合抑制和诱导细胞周期停滞的影响。几种类似物,其中包括10-(4-(3-甲氧基苯基)哌嗪-1-羰基)-10 H-吩恶嗪-3-腈(16o),显示出优异的抗增殖性能,低纳摩尔GI 50值(16o,平均胃肠道50(3.3 nM)对抗大量(93)癌细胞系。十五种化合物有效抑制微管蛋白聚合。通过流式细胞仪分析细胞周期发现,抑制肿瘤细胞生长与诱导G2 / M期细胞周期阻滞有关。蛋白质印迹和分子对接研究表明,这些化合物在秋水仙碱结合位点与β-微管蛋白有效结合。我们的研究证明了吩恶嗪和吩噻嗪核心以及苯基哌嗪部分对于开发新型有效的微管蛋白聚合抑制剂的适用性。
更新日期:2017-01-13
中文翻译:

衍生自吩恶嗪和吩噻嗪的N-杂环(4-苯基哌嗪-1-基)亚甲基酮作为微管蛋白聚合的高效抑制剂
我们在这里报告了一系列从三环杂环衍生的27 10-(4-苯基哌嗪-1-基)甲亚胺,这些环经筛选对肿瘤细胞生长,微管蛋白聚合抑制和诱导细胞周期停滞的影响。几种类似物,其中包括10-(4-(3-甲氧基苯基)哌嗪-1-羰基)-10 H-吩恶嗪-3-腈(16o),显示出优异的抗增殖性能,低纳摩尔GI 50值(16o,平均胃肠道50(3.3 nM)对抗大量(93)癌细胞系。十五种化合物有效抑制微管蛋白聚合。通过流式细胞仪分析细胞周期发现,抑制肿瘤细胞生长与诱导G2 / M期细胞周期阻滞有关。蛋白质印迹和分子对接研究表明,这些化合物在秋水仙碱结合位点与β-微管蛋白有效结合。我们的研究证明了吩恶嗪和吩噻嗪核心以及苯基哌嗪部分对于开发新型有效的微管蛋白聚合抑制剂的适用性。































 京公网安备 11010802027423号
京公网安备 11010802027423号