当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Structure of Murine N1-Acetylspermine Oxidase Reveals Molecular Details of Vertebrate Polyamine Catabolism
Biochemistry ( IF 2.9 ) Pub Date : 2017-01-12 00:00:00 , DOI: 10.1021/acs.biochem.6b01140 Tove Sjögren 1 , Carola M. Wassvik 2 , Arjan Snijder 1 , Anna Aagaard 1 , Taichi Kumanomidou 3 , Louise Barlind 1 , Tim P. Kaminski 1 , Akiko Kashima 3 , Takehiro Yokota 3 , Ola Fjellström 2
Biochemistry ( IF 2.9 ) Pub Date : 2017-01-12 00:00:00 , DOI: 10.1021/acs.biochem.6b01140 Tove Sjögren 1 , Carola M. Wassvik 2 , Arjan Snijder 1 , Anna Aagaard 1 , Taichi Kumanomidou 3 , Louise Barlind 1 , Tim P. Kaminski 1 , Akiko Kashima 3 , Takehiro Yokota 3 , Ola Fjellström 2
Affiliation
|
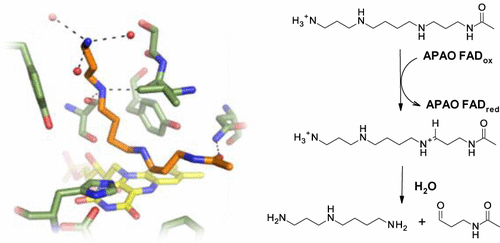
N1-Acetylspermine oxidase (APAO) catalyzes the conversion of N1-acetylspermine or N1-acetylspermidine to spermidine or putrescine, respectively, with concomitant formation of N-acetyl-3-aminopropanal and hydrogen peroxide. Here we present the structure of murine APAO in its oxidized holo form and in complex with substrate. The structures provide a basis for understanding molecular details of substrate interaction in vertebrate APAO, highlighting a key role for an asparagine residue in coordinating the N1-acetyl group of the substrate. We applied computational methods to the crystal structures to rationalize previous observations with regard to the substrate charge state. The analysis suggests that APAO features an active site ideally suited for binding of charged polyamines. We also reveal the structure of APAO in complex with the irreversible inhibitor MDL72527. In addition to the covalent adduct, a second MDL72527 molecule is bound in the active site. Binding of MDL72527 is accompanied by altered conformations in the APAO backbone. On the basis of structures of APAO, we discuss the potential for development of specific inhibitors.
中文翻译:

小鼠N 1-乙酰精胺氧化酶的结构揭示了脊椎动物多胺分解代谢的分子细节。
N 1-乙酰基精胺氧化酶(APAO)分别催化N 1-乙酰基精胺或N 1-乙酰基精胺向亚精胺或腐胺的转化,同时形成N-乙酰基-3-氨基丙醛和过氧化氢。在这里,我们介绍了氧化的全息形式并与底物复合的鼠类APAO的结构。该结构为理解脊椎动物APAO中底物相互作用的分子细节提供了基础,突出了天冬酰胺残基在协调N 1中的关键作用。底物的-乙酰基。我们将计算方法应用于晶体结构,以合理化先前关于衬底电荷状态的观察结果。分析表明,APAO具有一个理想的活性位点,适合结合带电荷的多胺。我们还揭示了与不可逆抑制剂MDL72527复合的APAO的结构。除了共价加合物以外,第二个MDL72527分子还结合在活性位点上。MDL72527的结合伴随着APAO主链中构象的改变。根据APAO的结构,我们讨论开发特定抑制剂的潜力。
更新日期:2017-01-12
中文翻译:

小鼠N 1-乙酰精胺氧化酶的结构揭示了脊椎动物多胺分解代谢的分子细节。
N 1-乙酰基精胺氧化酶(APAO)分别催化N 1-乙酰基精胺或N 1-乙酰基精胺向亚精胺或腐胺的转化,同时形成N-乙酰基-3-氨基丙醛和过氧化氢。在这里,我们介绍了氧化的全息形式并与底物复合的鼠类APAO的结构。该结构为理解脊椎动物APAO中底物相互作用的分子细节提供了基础,突出了天冬酰胺残基在协调N 1中的关键作用。底物的-乙酰基。我们将计算方法应用于晶体结构,以合理化先前关于衬底电荷状态的观察结果。分析表明,APAO具有一个理想的活性位点,适合结合带电荷的多胺。我们还揭示了与不可逆抑制剂MDL72527复合的APAO的结构。除了共价加合物以外,第二个MDL72527分子还结合在活性位点上。MDL72527的结合伴随着APAO主链中构象的改变。根据APAO的结构,我们讨论开发特定抑制剂的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号