当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Insights into the Thermodynamic Behavior of 2LiBH4–MgH2 Composite for Hydrogen Storage
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-06-30 00:00:00 , DOI: 10.1021/acs.jpcc.5b02047
Federico Cova 1 , Ewa C. E. Rönnebro 2 , Young Joon Choi 2, 3 , Fabiana C. Gennari 1 , Pierre Arneodo Larochette 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2015-06-30 00:00:00 , DOI: 10.1021/acs.jpcc.5b02047
Federico Cova 1 , Ewa C. E. Rönnebro 2 , Young Joon Choi 2, 3 , Fabiana C. Gennari 1 , Pierre Arneodo Larochette 1
Affiliation
|
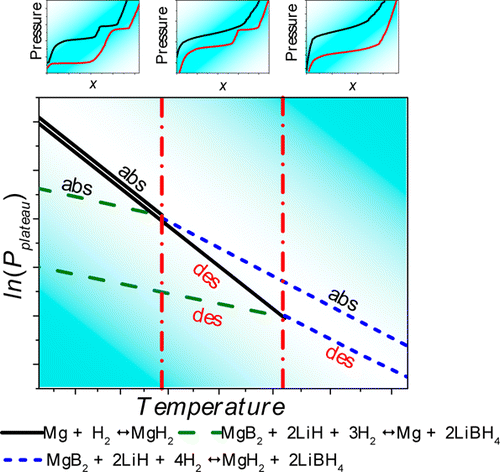
The composite 2LiBH4:MgH2 has been studied as a potential hydrogen storage material due to its high storage capacity. The present work is aimed at clarifying the thermodynamic behavior of the system, especially within the temperature region above 400 °C. Different reaction paths which have important implication for storage applications during hydrogen absorption and desorption at various temperatures were revealed. At temperatures over 413 °C, two different absorption pressure plateaus are observed. This indicates that two different reactions occur: Mg hydrogenation at higher pressures and the re-formation of LiBH4 from H2, LiH, and MgB2 at lower pressures. On the other hand, at temperatures below 413 °C only one plateau is present in the system. During desorption, the double plateau can be observed at temperatures as low as 375 °C. This effect restricts the applicability of this composite as a hydrogen storage material.
中文翻译:

新的洞察2LiBH的热力学行为4 -MgH 2复合储氢
复合材料2LiBH 4:MgH 2由于其高存储容量而被研究为潜在的储氢材料。本工作旨在阐明系统的热力学行为,尤其是在高于400°C的温度区域内。揭示了在不同温度下氢吸收和解吸期间对于存储应用具有重要意义的不同反应路径。在超过413°C的温度下,观察到两个不同的吸收压力平稳期。这表明发生了两种不同的反应:高压下的镁氢化和H 2,LiH和MgB 2从LiBH 4的重新形成在较低的压力下。另一方面,在低于413°C的温度下,系统中仅存在一个平稳段。在解吸过程中,可以在低至375°C的温度下观察到双平稳期。这种作用限制了该复合材料作为储氢材料的适用性。
更新日期:2015-06-30
中文翻译:

新的洞察2LiBH的热力学行为4 -MgH 2复合储氢
复合材料2LiBH 4:MgH 2由于其高存储容量而被研究为潜在的储氢材料。本工作旨在阐明系统的热力学行为,尤其是在高于400°C的温度区域内。揭示了在不同温度下氢吸收和解吸期间对于存储应用具有重要意义的不同反应路径。在超过413°C的温度下,观察到两个不同的吸收压力平稳期。这表明发生了两种不同的反应:高压下的镁氢化和H 2,LiH和MgB 2从LiBH 4的重新形成在较低的压力下。另一方面,在低于413°C的温度下,系统中仅存在一个平稳段。在解吸过程中,可以在低至375°C的温度下观察到双平稳期。这种作用限制了该复合材料作为储氢材料的适用性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号