当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of Hemiaminal Ethers by Chiral Brønsted Acids for Facile Access to Enantioselective Two‐Carbon Homologation Using Enecarbamates
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2009-03-05 , DOI: 10.1002/anie.200805385 Masahiro Terada , Kyoko Machioka , Keiichi Sorimachi
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2009-03-05 , DOI: 10.1002/anie.200805385 Masahiro Terada , Kyoko Machioka , Keiichi Sorimachi
|
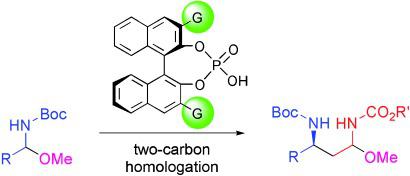
An enriching experience: Chiral phosphoric acids have been used to catalyze the title transformation for aromatic and aliphatic hemiaminal ethers. The process affords the corresponding products in good to high enantioselectivity (see scheme; Boc=tert‐butoxycarbonyl, G=aromatic group). The method enables facile access to highly enantioenriched 1,3‐diamine derivatives.

中文翻译:

手性布朗斯台德酸激活半胱氨酸醚,以方便地利用烯草胺类化合物获得对映选择性两碳同系物
丰富的经验:手性磷酸已被用于催化芳香族和脂肪族半乳糖醚的标题转化。该方法可提供具有良好至高对映选择性的相应产物(参见方案; Boc =叔丁氧羰基,G =芳族基团)。该方法可以轻松获得高度对映体富集的1,3-二胺衍生物。

更新日期:2009-03-05

中文翻译:

手性布朗斯台德酸激活半胱氨酸醚,以方便地利用烯草胺类化合物获得对映选择性两碳同系物
丰富的经验:手性磷酸已被用于催化芳香族和脂肪族半乳糖醚的标题转化。该方法可提供具有良好至高对映选择性的相应产物(参见方案; Boc =叔丁氧羰基,G =芳族基团)。该方法可以轻松获得高度对映体富集的1,3-二胺衍生物。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号