当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermochemical and Kinetics of CH3SH + H Reactions: The Sensitivity of Coupling the Low and High-Level Methodologies
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-01-06 00:00:00 , DOI: 10.1021/acs.jpca.6b09272
Daniely V. V. Cardoso 1 , Leonardo A. Cunha 1 , Rene F. K. Spada 2 , Corey A. Petty 1 , Luiz F. A. Ferrão 1 , Orlando Roberto-Neto 3 , Francisco B. C. Machado 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2017-01-06 00:00:00 , DOI: 10.1021/acs.jpca.6b09272
Daniely V. V. Cardoso 1 , Leonardo A. Cunha 1 , Rene F. K. Spada 2 , Corey A. Petty 1 , Luiz F. A. Ferrão 1 , Orlando Roberto-Neto 3 , Francisco B. C. Machado 1
Affiliation
|
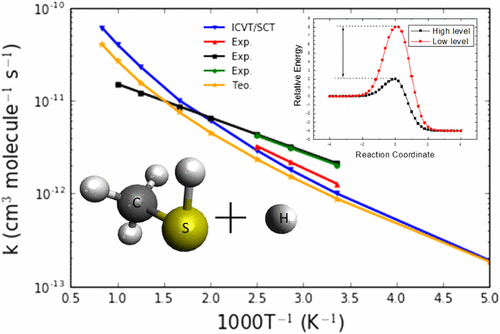
The reaction system formed by the methanethiol molecule (CH3SH) and a hydrogen atom was studied via three elementary reactions, two hydrogen abstractions and the C–S bond cleavage (CH3SH + H → CH3S + H2 (R1); → CH2SH + H2 (R2); → CH3 + H2S (R3)). The stable structures were optimized with various methodologies of the density functional theory and the MP2 method. Two minimum energy paths for each elementary reaction were built using the BB1K and MP2 methodologies, and the electronic properties on the reactants, products, and saddle points were improved with coupled cluster theory with single, double, and connected triple excitations (CCSD(T)) calculations. The sensitivity of coupling the low and high-level methods to calculate the thermochemical and rate constants were analyzed. The thermal rate constants were obtained by means of the improved canonical variational theory (ICVT) and the tunneling corrections were included with the small curvature tunneling (SCT) approach. Our results are in agreement with the previous experimental measurements and the calculated branching ratio for R1:R2:R3 is equal to 0.96:0:0.04, with kR1 = 9.64 × 10–13 cm3 molecule–1 s–1 at 298 K.
中文翻译:

CH 3 SH + H反应的热化学和动力学:低级和高级方法耦合的敏感性
甲烷硫醇分子(CH 3 SH)与氢原子形成的反应体系通过三个基本反应,两个氢抽象和C–S键裂解(CH 3 SH + H→CH 3 S + H 2(R1))进行了研究;→CH 2 SH + H 2(R2);→CH 3 + H 2S(R3))。用密度泛函理论和MP2方法的各种方法对稳定结构进行了优化。使用BB1K和MP2方法建立了每个基本反应的两条最小能量路径,并通过具有单,双和连接三重激发的耦合簇理论(CCSD(T))改善了反应物,产物和鞍点的电子性质。 )计算。分析了将低级方法和高级方法结合起来以计算热化学和速率常数的敏感性。借助改进的规范变分理论(ICVT)获得热速率常数,并且小曲率隧穿(SCT)方法包括隧穿校正。在298 K时k R1 = 9.64×10 –13 cm 3分子–1 s –1。
更新日期:2017-01-06
中文翻译:

CH 3 SH + H反应的热化学和动力学:低级和高级方法耦合的敏感性
甲烷硫醇分子(CH 3 SH)与氢原子形成的反应体系通过三个基本反应,两个氢抽象和C–S键裂解(CH 3 SH + H→CH 3 S + H 2(R1))进行了研究;→CH 2 SH + H 2(R2);→CH 3 + H 2S(R3))。用密度泛函理论和MP2方法的各种方法对稳定结构进行了优化。使用BB1K和MP2方法建立了每个基本反应的两条最小能量路径,并通过具有单,双和连接三重激发的耦合簇理论(CCSD(T))改善了反应物,产物和鞍点的电子性质。 )计算。分析了将低级方法和高级方法结合起来以计算热化学和速率常数的敏感性。借助改进的规范变分理论(ICVT)获得热速率常数,并且小曲率隧穿(SCT)方法包括隧穿校正。在298 K时k R1 = 9.64×10 –13 cm 3分子–1 s –1。

































 京公网安备 11010802027423号
京公网安备 11010802027423号