当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BF3·OEt2 Mediated Regioselective Reaction of Electron-Rich Arenes with 3-Ylidene Oxindoles
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-01-03 00:00:00 , DOI: 10.1021/acs.joc.6b02395
Nitika Sharma 1 , Rama Krishna Peddinti 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-01-03 00:00:00 , DOI: 10.1021/acs.joc.6b02395
Nitika Sharma 1 , Rama Krishna Peddinti 1
Affiliation
|
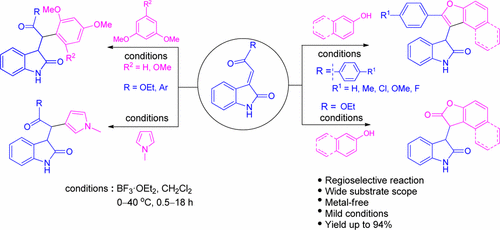
A BF3·OEt2 mediated novel and regioselective protocol for the construction of a C–C bond between 3-ylidene oxindoles and electron-rich arenes has been successfully accomplished. The reaction was compatible with a wide variety of electron-rich arenes. A cascade reaction of 3-ylidene oxindoles with phenols and β-naphthol resulted in 2,3-difunctionalized benzofuran and lactone bearing indoline-2-one scaffolds under same conditions.
中文翻译:

BF 3 ·OEt 2介导的电子富集芳烃与3-亚萘基氧杂环丁烷的区域选择性反应
已经成功完成了由BF 3 ·OEt 2介导的新颖的和区域选择性的方案,用于在3-亚甲基氧吲哚和富电子芳烃之间建立C–C键。该反应与多种富电子芳烃相容。在相同条件下,3-亚甲基羟吲哚与酚和β-萘酚的级联反应产生带有2,3-二官能化的苯并呋喃和内酯的二氢吲哚-2-酮骨架。
更新日期:2017-01-03
中文翻译:

BF 3 ·OEt 2介导的电子富集芳烃与3-亚萘基氧杂环丁烷的区域选择性反应
已经成功完成了由BF 3 ·OEt 2介导的新颖的和区域选择性的方案,用于在3-亚甲基氧吲哚和富电子芳烃之间建立C–C键。该反应与多种富电子芳烃相容。在相同条件下,3-亚甲基羟吲哚与酚和β-萘酚的级联反应产生带有2,3-二官能化的苯并呋喃和内酯的二氢吲哚-2-酮骨架。































 京公网安备 11010802027423号
京公网安备 11010802027423号