当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trimerization Reaction Kinetics and Tg Depression of Polycyanurate under Nanoconfinement
Macromolecules ( IF 5.1 ) Pub Date : 2015-06-29 00:00:00 , DOI: 10.1021/acs.macromol.5b00167
Evelyn Lopez 1 , Sindee L. Simon 1
Macromolecules ( IF 5.1 ) Pub Date : 2015-06-29 00:00:00 , DOI: 10.1021/acs.macromol.5b00167
Evelyn Lopez 1 , Sindee L. Simon 1
Affiliation
|
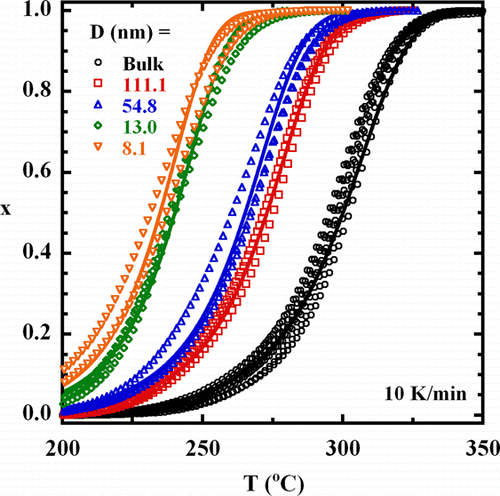
Trimerization of a mixture containing a mono- and difunctional cyanate ester is investigated under the nanoporous confinement of silanized hydrophobic controlled pore glass using differential scanning calorimetry. The trimerization reaction of the nanoconfined monomer mixture is accelerated relative to the bulk by as much as 12 times in 8 nm pores, but this acceleration is less than half that observed for nanoconfinement of the individual monomers. The absolute reaction rate of the monomer mixture lies between those of the individual species, being slower than the monocyanate ester and faster than the dicyanate ester. The results are consistent with the hypothesis that the reaction acceleration is due to monomer ordering or layering at the pore surface, leading to a local concentration of reactive groups higher than in the bulk. In addition to the influence of nanoconfinement on trimerization kinetics, the molecular weight and glass transition temperature (Tg) of the polycyanurate formed in the nanopores are investigated. The molecular weight decreases approximately 20% for synthesis in the smallest 8 nm pores relative to the bulk value of 5200 g/mol. Upon extraction from the pores, the polymer Tg is 5–9 K higher than in the bulk. However, in the 8 nm diameter pores, a Tg depression of 44 K is observed relative to the value of the material after extraction from the pores. This depression lies between the values previously observed for the products of the individual cyanate esters which formed a low molecular weight trimer and a cross-linked polymer network. A secondary Tg, associated with a less mobile layer at the pore wall, is 26–40 K above the primary value. The implication is that the origin of confinement effects on reactivity and Tg differ, with changes in reactivity in this system arising from surface layering or ordering and Tg depressions arising from intrinsic size effects.
中文翻译:

纳米约束下三聚化反应动力学和聚氰尿酸酯的T g降低
使用差示扫描量热法在硅烷化疏水控制孔玻璃的纳米孔约束下研究了含有单官能和双官能氰酸酯的混合物的三聚化。纳米约束单体混合物的三聚反应在8 nm的孔中相对于本体加速了12倍,但该加速度小于单个单体纳米约束所观察到的一半。单体混合物的绝对反应速率介于各个物种的绝对反应速率之间,比单氰酸酯酯慢并且比二氰酸酯酯快。该结果与以下假设一致:反应加速是由于单体在孔表面有序排列或分层,导致反应性基团的局部浓度高于本体中的浓度。研究了在纳米孔中形成的聚氰尿酸酯的T g)。相对于5200 g / mol的体积值,在最小的8 nm孔中合成时,分子量降低了约20%。从孔中提取后,聚合物的T g比本体中的聚合物高5–9K。但是,在直径为8 nm的孔中,相对于从孔中提取后的材料值,观察到T g降低了44K。这种降低介于先前观察到的形成低分子量三聚体和交联聚合物网络的各个氰酸酯产物的值之间。二次T g,与孔壁上流动性较弱的层相关,比初始值高26–40K。这意味着约束作用对反应性和T g的起因是不同的,该系统中反应性的变化是由于表面分层或有序产生的,而T g凹陷是由于内在的尺寸效应引起的。
更新日期:2015-06-29
中文翻译:

纳米约束下三聚化反应动力学和聚氰尿酸酯的T g降低
使用差示扫描量热法在硅烷化疏水控制孔玻璃的纳米孔约束下研究了含有单官能和双官能氰酸酯的混合物的三聚化。纳米约束单体混合物的三聚反应在8 nm的孔中相对于本体加速了12倍,但该加速度小于单个单体纳米约束所观察到的一半。单体混合物的绝对反应速率介于各个物种的绝对反应速率之间,比单氰酸酯酯慢并且比二氰酸酯酯快。该结果与以下假设一致:反应加速是由于单体在孔表面有序排列或分层,导致反应性基团的局部浓度高于本体中的浓度。研究了在纳米孔中形成的聚氰尿酸酯的T g)。相对于5200 g / mol的体积值,在最小的8 nm孔中合成时,分子量降低了约20%。从孔中提取后,聚合物的T g比本体中的聚合物高5–9K。但是,在直径为8 nm的孔中,相对于从孔中提取后的材料值,观察到T g降低了44K。这种降低介于先前观察到的形成低分子量三聚体和交联聚合物网络的各个氰酸酯产物的值之间。二次T g,与孔壁上流动性较弱的层相关,比初始值高26–40K。这意味着约束作用对反应性和T g的起因是不同的,该系统中反应性的变化是由于表面分层或有序产生的,而T g凹陷是由于内在的尺寸效应引起的。







































 京公网安备 11010802027423号
京公网安备 11010802027423号