当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tris(pentafluorophenyl)borane‐Catalyzed Reaction of Phosphorus/Boron and Nitrogen/Boron Frustrated Lewis Pair Dihydrogen Activation Products with Alkenes and Alkynes
ChemCatChem ( IF 3.8 ) Pub Date : 2017-01-03 , DOI: 10.1002/cctc.201601229 Tongdao Wang 1 , Xenia Jentgens 1 , Constantin G. Daniliuc 1 , Gerald Kehr 1 , Gerhard Erker 1
ChemCatChem ( IF 3.8 ) Pub Date : 2017-01-03 , DOI: 10.1002/cctc.201601229 Tongdao Wang 1 , Xenia Jentgens 1 , Constantin G. Daniliuc 1 , Gerald Kehr 1 , Gerhard Erker 1
Affiliation
|
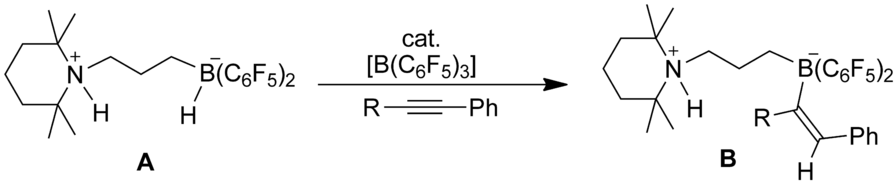
The N/B frustrated Lewis pair (FLP) dihydrogen splitting product 10 undergoes a facile B(C6F5)3‐catalyzed regioselective trans‐1,2‐hydridoborate addition reaction to phenylacetylene to give the cis‐styryl‐containing ammonium/borate zwitterion 12. The B(C6F5)3‐catalyzed reaction of 10 with 1‐phenylpropyne proceeded analogously to give 13. The respective [B]/H− addition to styrene gave the corresponding [B]−CH2CH2Ph‐containing product 14 under mild conditions. We obtained evidence that the reaction is initiated by hydride migration between the two B centers involved and is then continued by hydride addition to the borane activated alkyne or alkene, respectively. The H2‐splitting product 4 derived from the ethylene‐bridged P/B FLP 3 reacted analogously with these alkynes and also with styrene under B(C6F5)3 catalysis to yield the respective alkenyl‐ or alkyl borate products. The new addition products were characterized by using XRD.
中文翻译:

三(五氟苯基)硼烷催化的磷/硼和氮/硼受阻路易斯对双氢活化产物与烯烃和炔烃的反应
N / B沮丧的路易斯对(FLP)二氢裂解产物10经历了简便的B(C 6 F 5)3催化的对苯乙炔的区域选择性反式1,2-氢硼酸酯加成反应,从而生成含顺式-苯乙烯基的铵/硼酸酯两性离子12。10与1-苯基丙炔的B(C 6 F 5)3催化反应类似地进行,得到13。各自的[B] / H -除了苯乙烯,得到相应的[B] -CH 2 CH 2含有PH-产物14在温和的条件下。我们获得的证据表明,反应是由涉及的两个B中心之间的氢化物迁移引发的,然后通过分别向硼烷活化的炔烃或烯烃中氢化物的添加而继续进行。在B(C 6 F 5)3催化下,由乙烯桥连的P / B FLP 3衍生的H 2分解产物4与这些炔烃以及与苯乙烯类似地反应,生成相应的烯基硼酸或烷基硼酸酯产物。通过使用XRD对新添加的产品进行了表征。
更新日期:2017-01-03
中文翻译:

三(五氟苯基)硼烷催化的磷/硼和氮/硼受阻路易斯对双氢活化产物与烯烃和炔烃的反应
N / B沮丧的路易斯对(FLP)二氢裂解产物10经历了简便的B(C 6 F 5)3催化的对苯乙炔的区域选择性反式1,2-氢硼酸酯加成反应,从而生成含顺式-苯乙烯基的铵/硼酸酯两性离子12。10与1-苯基丙炔的B(C 6 F 5)3催化反应类似地进行,得到13。各自的[B] / H -除了苯乙烯,得到相应的[B] -CH 2 CH 2含有PH-产物14在温和的条件下。我们获得的证据表明,反应是由涉及的两个B中心之间的氢化物迁移引发的,然后通过分别向硼烷活化的炔烃或烯烃中氢化物的添加而继续进行。在B(C 6 F 5)3催化下,由乙烯桥连的P / B FLP 3衍生的H 2分解产物4与这些炔烃以及与苯乙烯类似地反应,生成相应的烯基硼酸或烷基硼酸酯产物。通过使用XRD对新添加的产品进行了表征。































 京公网安备 11010802027423号
京公网安备 11010802027423号