当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visualizing the Cu/Cu2O Interface Transition in Nanoparticles with Environmental Scanning Transmission Electron Microscopy
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-12-27 , DOI: 10.1021/jacs.6b08842
Alec P. LaGrow 1 , Michael R. Ward 1 , David C. Lloyd 1 , Pratibha L. Gai 1 , Edward D. Boyes 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-12-27 , DOI: 10.1021/jacs.6b08842
Alec P. LaGrow 1 , Michael R. Ward 1 , David C. Lloyd 1 , Pratibha L. Gai 1 , Edward D. Boyes 1
Affiliation
|
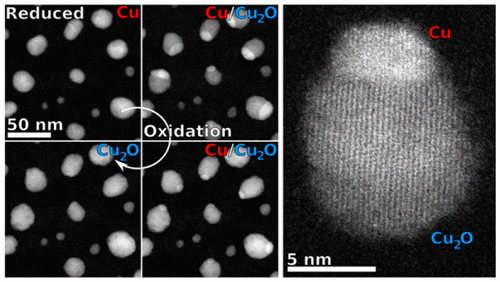
Understanding the oxidation and reduction mechanisms of catalytically active transition metal nanoparticles is important to improve their application in a variety of chemical processes. In nanocatalysis the nanoparticles can undergo oxidation or reduction in situ, and thus the redox species are not what are observed before and after reactions. We have used the novel environmental scanning transmission electron microscope (ESTEM) with 0.1 nm resolution in systematic studies of complex dynamic oxidation and reduction mechanisms of copper nanoparticles. The oxidation of copper has previously been reported to be dependent on its crystallography and its interaction with the substrate. By following the dynamic oxidation process in situ in real time with high-angle annular dark-field imaging in the ESTEM, we use conditions ideal to track the oxidation front as it progresses across a copper nanoparticle by following the changes in the atomic number (Z) contrast with time. The oxidation occurs via the nucleation of the oxide phase (Cu2O) from one area of the nanoparticle which then progresses unidirectionally across the particle, with the Cu-to-Cu2O interface having a relationship of Cu{111}//Cu2O{111}. The oxidation kinetics are related to the temperature and oxygen pressure. When the process is reversed in hydrogen, the reduction process is observed to be similar to the oxidation, with the same crystallographic relationship between the two phases. The dynamic observations provide unique insights into redox mechanisms which are important to understanding and controlling the oxidation and reduction of copper-based nanoparticles.
中文翻译:

使用环境扫描透射电子显微镜观察纳米颗粒中的 Cu/Cu2O 界面转变
了解具有催化活性的过渡金属纳米粒子的氧化和还原机制对于改进它们在各种化学过程中的应用很重要。在纳米催化中,纳米粒子可以在原位进行氧化或还原,因此在反应前后观察不到氧化还原物质。我们使用分辨率为 0.1 nm 的新型环境扫描透射电子显微镜 (ESTEM) 系统研究了铜纳米粒子的复杂动态氧化和还原机制。先前已报道铜的氧化取决于其晶体学及其与基材的相互作用。通过在 ESTEM 中使用高角度环形暗场成像实时跟踪原位动态氧化过程,我们使用理想的条件来跟踪氧化前沿,因为它通过跟踪原子序数 (Z) 随时间的变化而在铜纳米颗粒上进行。氧化通过来自纳米颗粒一个区域的氧化物相 (Cu2O) 的成核发生,然后单向穿过颗粒,Cu 与 Cu2O 的界面具有 Cu{111}//Cu2O{111} 的关系。氧化动力学与温度和氧气压力有关。当该过程在氢气中逆转时,观察到还原过程类似于氧化,两相之间具有相同的晶体学关系。动态观察提供了对氧化还原机制的独特见解,这对于理解和控制铜基纳米粒子的氧化和还原很重要。
更新日期:2016-12-27
中文翻译:

使用环境扫描透射电子显微镜观察纳米颗粒中的 Cu/Cu2O 界面转变
了解具有催化活性的过渡金属纳米粒子的氧化和还原机制对于改进它们在各种化学过程中的应用很重要。在纳米催化中,纳米粒子可以在原位进行氧化或还原,因此在反应前后观察不到氧化还原物质。我们使用分辨率为 0.1 nm 的新型环境扫描透射电子显微镜 (ESTEM) 系统研究了铜纳米粒子的复杂动态氧化和还原机制。先前已报道铜的氧化取决于其晶体学及其与基材的相互作用。通过在 ESTEM 中使用高角度环形暗场成像实时跟踪原位动态氧化过程,我们使用理想的条件来跟踪氧化前沿,因为它通过跟踪原子序数 (Z) 随时间的变化而在铜纳米颗粒上进行。氧化通过来自纳米颗粒一个区域的氧化物相 (Cu2O) 的成核发生,然后单向穿过颗粒,Cu 与 Cu2O 的界面具有 Cu{111}//Cu2O{111} 的关系。氧化动力学与温度和氧气压力有关。当该过程在氢气中逆转时,观察到还原过程类似于氧化,两相之间具有相同的晶体学关系。动态观察提供了对氧化还原机制的独特见解,这对于理解和控制铜基纳米粒子的氧化和还原很重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号