当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings.
Nature Communications ( IF 14.7 ) Pub Date : 2016-12-22 , DOI: 10.1038/ncomms13852
Lei Li 1 , Zhong-Liang Li 1 , Fu-Li Wang 1 , Zhen Guo 2 , Yong-Feng Cheng 1 , Na Wang 2 , Xiao-Wu Dong 3 , Chao Fang 4 , Jingjiang Liu 4 , Chunhui Hou 4 , Bin Tan 1 , Xin-Yuan Liu 1
Nature Communications ( IF 14.7 ) Pub Date : 2016-12-22 , DOI: 10.1038/ncomms13852
Lei Li 1 , Zhong-Liang Li 1 , Fu-Li Wang 1 , Zhen Guo 2 , Yong-Feng Cheng 1 , Na Wang 2 , Xiao-Wu Dong 3 , Chao Fang 4 , Jingjiang Liu 4 , Chunhui Hou 4 , Bin Tan 1 , Xin-Yuan Liu 1
Affiliation
|
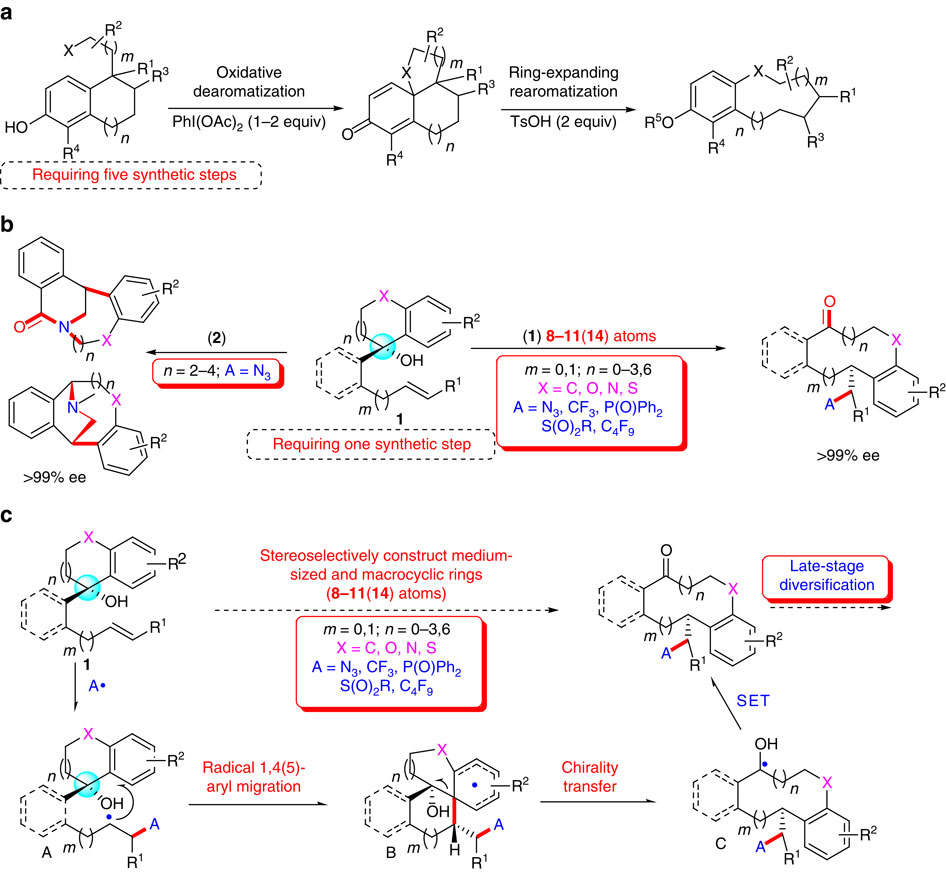
Medium-sized and medium-bridged rings are attractive structural motifs in natural products and therapeutic agents. Due to the unfavourable entropic and/or enthalpic factors with these ring systems, their efficient construction remains a formidable challenge. To address this problem, we herein disclose a radical-based approach for diversity-oriented synthesis of various benzannulated carbon- and heteroatom-containing 8-11(14)-membered ketone libraries. This strategy involves 1,4- or 1,5-aryl migration triggered by radical azidation, trifluoromethylation, phosphonylation, sulfonylation, or perfluoroalkylation of unactivated alkenes followed by intramolecular ring expansion. Demonstration of this method as a highly flexible tool for the construction of 37 synthetically challenging medium-sized and macrocyclic ring scaffolds including bridged rings with diverse functionalities and skeletons is highlighted. Some of these products showed potent inhibitory activity against the cancer cell or derivative of human embryonic kidney line in preliminary biological studies. The mechanism of this novel strategy is investigated by control experiments and DFT calculations.
中文翻译:

自由基芳基迁移能够以多样性为导向合成结构多样的中环/大环或桥环。
中等尺寸和中等桥环是天然产物和治疗剂中有吸引力的结构图案。由于这些环系统存在不利的熵和/或焓因素,它们的有效构建仍然是一个艰巨的挑战。为了解决这个问题,我们在此公开了一种基于自由基的方法,用于以多样性为导向合成各种苯并环化的含碳和杂原子的8-11(14)元酮库。该策略涉及由未活化烯烃的自由基叠氮化、三氟甲基化、膦酰化、磺酰化或全氟烷基化引发的1,4-或1,5-芳基迁移,随后进行分子内环扩展。重点展示了该方法作为高度灵活的工具,用于构建 37 个具有综合挑战性的中型和大环支架,包括具有不同功能和骨架的桥环。在初步生物学研究中,其中一些产品显示出对癌细胞或人胚胎肾系衍生物的有效抑制活性。通过控制实验和 DFT 计算研究了这种新策略的机制。
更新日期:2016-12-23
中文翻译:

自由基芳基迁移能够以多样性为导向合成结构多样的中环/大环或桥环。
中等尺寸和中等桥环是天然产物和治疗剂中有吸引力的结构图案。由于这些环系统存在不利的熵和/或焓因素,它们的有效构建仍然是一个艰巨的挑战。为了解决这个问题,我们在此公开了一种基于自由基的方法,用于以多样性为导向合成各种苯并环化的含碳和杂原子的8-11(14)元酮库。该策略涉及由未活化烯烃的自由基叠氮化、三氟甲基化、膦酰化、磺酰化或全氟烷基化引发的1,4-或1,5-芳基迁移,随后进行分子内环扩展。重点展示了该方法作为高度灵活的工具,用于构建 37 个具有综合挑战性的中型和大环支架,包括具有不同功能和骨架的桥环。在初步生物学研究中,其中一些产品显示出对癌细胞或人胚胎肾系衍生物的有效抑制活性。通过控制实验和 DFT 计算研究了这种新策略的机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号