当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Band Gap Relationships in Hexagonal Polytypes and Low-Dimensional Structures of Hybrid Tin Iodide Perovskites
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2016-12-20 00:00:00 , DOI: 10.1021/acs.inorgchem.6b02764 Constantinos C. Stoumpos 1 , Lingling Mao 1 , Christos D. Malliakas 1 , Mercouri G. Kanatzidis 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2016-12-20 00:00:00 , DOI: 10.1021/acs.inorgchem.6b02764 Constantinos C. Stoumpos 1 , Lingling Mao 1 , Christos D. Malliakas 1 , Mercouri G. Kanatzidis 1
Affiliation
|
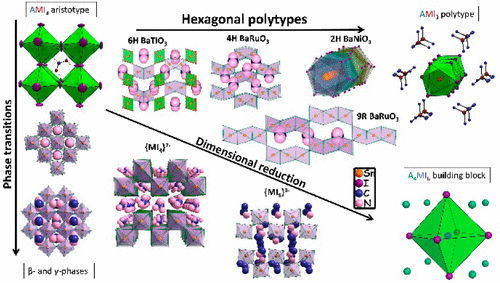
The present study deals with the structural characterization and classification of the novel compounds 1–8 into perovskite subclasses and proceeds in extracting the structure–band gap relationships between them. The compounds were obtained from the employment of small, 3–5-atom-wide organic ammonium ions seeking to discover new perovskite-like compounds. The compounds reported here adopt unique or rare structure types akin to the prototype structure perovskite. When trimethylammonium (TMA) was employed, we obtained TMASnI3 (1), which is our reference compound for a “perovskitoid” structure of face-sharing octahedra. The compounds EASnI3 (2b), GASnI3 (3a), ACASnI3 (4), and IMSnI3 (5) obtained from the use of ethylammonium (EA), guanidinium (GA), acetamidinium (ACA), and imidazolium (IM) cations, respectively, represent the first entries of the so-called “hexagonal perovskite polytypes” in the hybrid halide perovskite library. The hexagonal perovskites define a new family of hybrid halide perovskites with a crystal structure that emerges from a blend of corner- and face-sharing octahedral connections in various proportions. The small organic cations can also stabilize a second structural type characterized by a crystal lattice with reduced dimensionality. These compounds include the two-dimensional (2D) perovskites GA2SnI4 (3b) and IPA3Sn2I7 (6b) and the one-dimensional (1D) perovskite IPA3SnI5 (6a). The known 2D perovskite BA2MASn2I7 (7) and the related all-inorganic 1D perovskite “RbSnF2I” (8) have also been synthesized. All compounds have been identified as medium-to-wide-band-gap semiconductors in the range of Eg = 1.90–2.40 eV, with the band gap progressively decreasing with increased corner-sharing functionality and increased torsion angle in the octahedral connectivity.
中文翻译:

杂化碘化钙钛矿的六角多型与低维结构的结构带隙关系
本研究中涉及的新的化合物的结构表征和分类1 - 8到钙钛矿的子类和在提取它们之间的结构带隙的关系进行。这些化合物是通过使用3至5个原子宽的有机铵小离子而获得的,这些离子试图发现类似钙钛矿的新化合物。本文报道的化合物采用与钙钛矿原型结构相似的独特或稀有结构类型。当使用三甲基铵(TMA)时,我们获得了TMASnI 3(1),它是面部共享八面体“钙钛矿型”结构的参考化合物。化合物EASnI 3(2b),GASnI 3(3a),ACASnI 3(4)和IMSnI 3(5)分别是通过使用乙铵(EA),胍(GA),乙AC (ACA)和咪唑(IM)阳离子获得的。杂化卤化物钙钛矿文库中的所谓“六方钙钛矿多型”。六角形钙钛矿定义了杂化卤化物钙钛矿的新家族,其晶体结构是由角和面共享八面体连接以不同比例混合而成的。较小的有机阳离子还可以使第二结构类型稳定,该第二结构类型的特征在于具有减小的尺寸的晶格。这些化合物包括二维(2D)钙钛矿GA 2 SnI 4。(3b)和IPA 3 Sn 2 I 7(6b)和一维(1D)钙钛矿IPA 3 SnI 5(6a)。还合成了已知的2D钙钛矿BA 2 MASn 2 I 7(7)和相关的全无机1D钙钛矿“ RbSnF 2 I”(8)。所有化合物都被确定为E g范围内的中到宽带隙半导体 = 1.90–2.40 eV,随着带角共享功能的增加和八面体连接中的扭转角的增加,带隙逐渐减小。
更新日期:2016-12-20
中文翻译:

杂化碘化钙钛矿的六角多型与低维结构的结构带隙关系
本研究中涉及的新的化合物的结构表征和分类1 - 8到钙钛矿的子类和在提取它们之间的结构带隙的关系进行。这些化合物是通过使用3至5个原子宽的有机铵小离子而获得的,这些离子试图发现类似钙钛矿的新化合物。本文报道的化合物采用与钙钛矿原型结构相似的独特或稀有结构类型。当使用三甲基铵(TMA)时,我们获得了TMASnI 3(1),它是面部共享八面体“钙钛矿型”结构的参考化合物。化合物EASnI 3(2b),GASnI 3(3a),ACASnI 3(4)和IMSnI 3(5)分别是通过使用乙铵(EA),胍(GA),乙AC (ACA)和咪唑(IM)阳离子获得的。杂化卤化物钙钛矿文库中的所谓“六方钙钛矿多型”。六角形钙钛矿定义了杂化卤化物钙钛矿的新家族,其晶体结构是由角和面共享八面体连接以不同比例混合而成的。较小的有机阳离子还可以使第二结构类型稳定,该第二结构类型的特征在于具有减小的尺寸的晶格。这些化合物包括二维(2D)钙钛矿GA 2 SnI 4。(3b)和IPA 3 Sn 2 I 7(6b)和一维(1D)钙钛矿IPA 3 SnI 5(6a)。还合成了已知的2D钙钛矿BA 2 MASn 2 I 7(7)和相关的全无机1D钙钛矿“ RbSnF 2 I”(8)。所有化合物都被确定为E g范围内的中到宽带隙半导体 = 1.90–2.40 eV,随着带角共享功能的增加和八面体连接中的扭转角的增加,带隙逐渐减小。































 京公网安备 11010802027423号
京公网安备 11010802027423号