当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the Reaction Mechanism of CO2 Electroreduction over Ag Films via Operando Infrared Spectroscopy
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-12-20 00:00:00 , DOI: 10.1021/acscatal.6b02382 Nienke J. Firet 1 , Wilson A. Smith 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2016-12-20 00:00:00 , DOI: 10.1021/acscatal.6b02382 Nienke J. Firet 1 , Wilson A. Smith 1
Affiliation
|
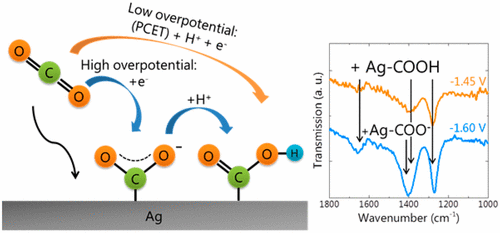
The electrocatalytic reduction of CO2 to chemical fuels has attracted significant attention in recent years. Among transition metals, silver shows one of the highest faradaic efficiencies for CO formation as the main reaction product; however, the exact mechanism for this conversion is not fully understood. In this work, we study the reaction mechanism of silver as a CO2 reduction catalyst using in situ attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) during electrochemical cycling. Using ATR-FTIR, it is possible to observe the reaction intermediates on the surface of Ag thin films formed during the CO2 electroreduction reaction. At a moderate overpotential, a proton coupled electron transfer reaction mechanism is confirmed to be the dominant CO2 reduction pathway. However, at a more negative applied potential, both the COO– and the COOH intermediates are detected using ATR-FTIR, which indicates that individual proton and electron transfer steps occur, offering a different pathway than at lower potentials. These results indicate that the CO2 reduction reaction mechanism can be potential dependent and not always involving a concerted proton coupled electron transfer, opening alternative pathways to optimize efficient and selective catalysts for desired product formation.
中文翻译:

用操作红外光谱探测银膜上CO 2的电还原反应机理
近年来,将CO 2电催化还原为化学燃料引起了广泛的关注。在过渡金属中,作为主要反应产物,银显示出最高的法拉第效率。但是,这种转换的确切机制尚未完全了解。在这项工作中,我们在电化学循环过程中使用原位衰减全反射傅里叶变换红外光谱(ATR-FTIR)研究了银作为CO 2还原催化剂的反应机理。使用ATR-FTIR,可以观察到CO 2形成的Ag薄膜表面的反应中间体电还原反应。在中等的超电势下,质子偶联的电子转移反应机制被证实是主要的CO 2还原途径。但是,在施加更大负电势时,使用ATR-FTIR可以检测到COO –和COOH中间体,这表明发生了单独的质子和电子转移步骤,与较低电势提供了不同的途径。这些结果表明,CO 2还原反应机理可能是电势依赖性的,并不总是涉及协调的质子耦合电子转移,从而打开了替代途径,以优化用于所需产物形成的高效和选择性催化剂。
更新日期:2016-12-20
中文翻译:

用操作红外光谱探测银膜上CO 2的电还原反应机理
近年来,将CO 2电催化还原为化学燃料引起了广泛的关注。在过渡金属中,作为主要反应产物,银显示出最高的法拉第效率。但是,这种转换的确切机制尚未完全了解。在这项工作中,我们在电化学循环过程中使用原位衰减全反射傅里叶变换红外光谱(ATR-FTIR)研究了银作为CO 2还原催化剂的反应机理。使用ATR-FTIR,可以观察到CO 2形成的Ag薄膜表面的反应中间体电还原反应。在中等的超电势下,质子偶联的电子转移反应机制被证实是主要的CO 2还原途径。但是,在施加更大负电势时,使用ATR-FTIR可以检测到COO –和COOH中间体,这表明发生了单独的质子和电子转移步骤,与较低电势提供了不同的途径。这些结果表明,CO 2还原反应机理可能是电势依赖性的,并不总是涉及协调的质子耦合电子转移,从而打开了替代途径,以优化用于所需产物形成的高效和选择性催化剂。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号