Nature Chemistry ( IF 19.2 ) Pub Date : 2016-12-19 , DOI: 10.1038/nchem.2682 Rasmus Mose , Gert Preegel , Jesper Larsen , Sofie Jakobsen , Eva Høgh Iversen , Karl Anker Jørgensen
|
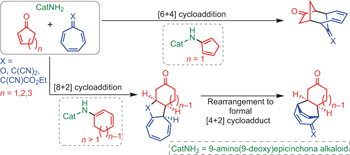
Cycloadditions that involve more than six π electrons are termed higher-order cycloadditions and are an excellent tool for solving complex synthetic challenges, as they provide direct access to polycyclic scaffolds that contain medium-sized rings. They have interesting synthetic potential for the discovery of new bioactive molecules and in natural product synthesis. It is peculiar that stereocontrolled [8+2] and [6+4] cycloadditions have been largely neglected for the past 50 years. Here we demonstrate a cross-dienamine activation of 2-cyclopentenone and the unprecedented endocyclic linear-dienamine activation of 2-cyclohexenones and 2-cycloheptenones. These dienamine intermediates undergo aminocatalytic stereoselective [8+2], [6+4] and formal [4+2] cycloadditions with various heptafulvenes. The periselectivities of the cycloadditions are controlled based on the ring size of the 2-cycloalkenones and the substitution patterns of the heptafulvenes. The chiral products obtained undergo various chemical and photochemical single-step transformations that give access to other classes of all-carbon polycyclic scaffolds.
中文翻译:

有机催化立体选择性[8 + 2]和[6 + 4]环加成
涉及超过六个π的环加成电子被称为高阶环加成,是解决复杂的合成难题的绝佳工具,因为它们可以直接进入含有中型环的多环支架。它们对于发现新的生物活性分子和天然产物合成具有有趣的合成潜力。在过去的50年中,立体声控制的[8 + 2]和[6 + 4]环加成被忽略了,这是很奇怪的。在这里,我们证明了2-环戊烯酮的跨二烯胺活化和2-环己烯酮和2-环庚烯酮的空前的环内线性二烯胺活化。这些二烯胺中间体与各种七氟戊烯进行氨基催化的立体选择性[8 + 2],[6 + 4]和正式的[4 + 2]环加成反应。基于2-环烯酮的环大小和七烯酮的取代模式来控制环加成的选择性。所获得的手性产物经历了各种化学和光化学单步转化,从而可以进入其他类别的全碳多环支架。






























 京公网安备 11010802027423号
京公网安备 11010802027423号