当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical vs Electrochemical Formation of Li2CO3 as a Discharge Product in Li–O2/CO2 Batteries by Controlling the Superoxide Intermediate
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2016-12-19 00:00:00 , DOI: 10.1021/acs.jpclett.6b02610
Wei Yin 1, 2 , Alexis Grimaud 1, 3 , Florent Lepoivre 1, 2 , Chunzhen Yang 1 , Jean Marie Tarascon 1, 2, 3, 4
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2016-12-19 00:00:00 , DOI: 10.1021/acs.jpclett.6b02610
Wei Yin 1, 2 , Alexis Grimaud 1, 3 , Florent Lepoivre 1, 2 , Chunzhen Yang 1 , Jean Marie Tarascon 1, 2, 3, 4
Affiliation
|
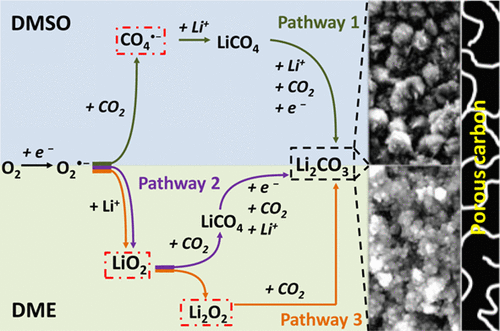
The Li–O2/CO2 battery with high capacity has recently been proposed as a new protocol to convert CO2. However, the fundamental mechanism for the reaction still remains hazy. Here, we investigated the discharge processes of Li–O2/CO2 (70%/30%) batteries in two solvents, dimethyl sulfoxide (DMSO) and 1,2-dimethoxyethane (DME). During discharge, both solvents initially show the reduction of oxygen. However, afterward, the solvent affects the reaction pathways of superoxide species by solvating Li+ with different strength, depending on the so-called donor number. More precisely, the initial formation of CO4•– is favored in DMSO at the expense of lithium superoxide formation that we observed in DME. Despite the different intermediate processes, X-ray diffraction showed that Li2CO3 was the final discharge product in both solvents. Moreover, we observed that CO2 cannot be reduced within the electrochemical stability window of DMSO and DME.
中文翻译:

通过控制超氧化物中间产物在Li–O 2 / CO 2电池中放电产物Li 2 CO 3的化学与电化学形成
高容量的Li–O 2 / CO 2电池最近被提出作为转换CO 2的新协议。但是,反应的基本机理仍然不明确。在这里,我们研究了Li–O 2 / CO 2(70%/ 30%)电池在二甲亚砜(DMSO)和1,2-二甲氧基乙烷(DME)两种溶剂中的放电过程。在放电过程中,两种溶剂最初都会显示出氧气的减少。然而,此后,取决于所谓的给体数,溶剂通过使具有不同强度的Li +溶剂化而影响超氧化物类的反应途径。更确切地说,CO 4 •的初始形成如我们在DME中观察到的那样,在DMSO中偏爱于以形成过氧化锂为代价。尽管中间过程不同,但X射线衍射显示Li 2 CO 3是两种溶剂中的最终放电产物。此外,我们观察到在DMSO和DME的电化学稳定性窗口内无法还原CO 2。
更新日期:2016-12-19
中文翻译:

通过控制超氧化物中间产物在Li–O 2 / CO 2电池中放电产物Li 2 CO 3的化学与电化学形成
高容量的Li–O 2 / CO 2电池最近被提出作为转换CO 2的新协议。但是,反应的基本机理仍然不明确。在这里,我们研究了Li–O 2 / CO 2(70%/ 30%)电池在二甲亚砜(DMSO)和1,2-二甲氧基乙烷(DME)两种溶剂中的放电过程。在放电过程中,两种溶剂最初都会显示出氧气的减少。然而,此后,取决于所谓的给体数,溶剂通过使具有不同强度的Li +溶剂化而影响超氧化物类的反应途径。更确切地说,CO 4 •的初始形成如我们在DME中观察到的那样,在DMSO中偏爱于以形成过氧化锂为代价。尽管中间过程不同,但X射线衍射显示Li 2 CO 3是两种溶剂中的最终放电产物。此外,我们观察到在DMSO和DME的电化学稳定性窗口内无法还原CO 2。

































 京公网安备 11010802027423号
京公网安备 11010802027423号