当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Hydrofluoroether Cosolvent Addition on Li Solvation in Acetonitrile-Based Solvate Electrolytes and Its Influence on S Reduction in a Li–S Battery
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2016-12-08 00:00:00 , DOI: 10.1021/acsami.6b11358
Kimberly A. See 1, 2 , Heng-Liang Wu 1, 2 , Kah Chun Lau 1, 3 , Minjeong Shin 1, 2 , Lei Cheng 1 , Mahalingam Balasubramanian 1 , Kevin G. Gallagher 1 , Larry A. Curtiss 1 , Andrew A. Gewirth 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2016-12-08 00:00:00 , DOI: 10.1021/acsami.6b11358
Kimberly A. See 1, 2 , Heng-Liang Wu 1, 2 , Kah Chun Lau 1, 3 , Minjeong Shin 1, 2 , Lei Cheng 1 , Mahalingam Balasubramanian 1 , Kevin G. Gallagher 1 , Larry A. Curtiss 1 , Andrew A. Gewirth 1, 2
Affiliation
|
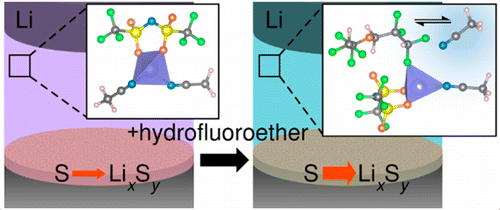
Li–S batteries are a promising next-generation battery technology. Due to the formation of soluble polysulfides during cell operation, the electrolyte composition of the cell plays an active role in directing the formation and speciation of the soluble lithium polysulfides. Recently, new classes of electrolytes termed “solvates” that contain stoichiometric quantities of salt and solvent and form a liquid at room temperature have been explored due to their sparingly solvating properties with respect to polysulfides. The viscosity of the solvate electrolytes is understandably high limiting their viability; however, hydrofluoroether cosolvents, thought to be inert to the solvate structure itself, can be introduced to reduce viscosity and enhance diffusion. Nazar and co-workers previously reported that addition of 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether (TTE) to the LiTFSI in acetonitrile solvate, (MeCN)2–LiTFSI, results in enhanced capacity retention compared to the neat solvate. Here, we evaluate the effect of TTE addition on both the electrochemical behavior of the Li–S cell and the solvation structure of the (MeCN)2–LiTFSI electrolyte. Contrary to previous suggestions, Raman and NMR spectroscopy coupled with ab initio molecular dynamics simulations show that TTE coordinates to Li+ at the expense of MeCN coordination, thereby producing a higher content of free MeCN, a good polysulfide solvent, in the electrolyte. The electrolytes containing a higher free MeCN content facilitate faster polysulfide formation kinetics during the electrochemical reduction of S in a Li–S cell likely as a result of the solvation power of the free MeCN.
中文翻译:

氢氟醚助溶剂的添加对乙腈基溶剂化物中Li溶剂化的影响及其对Li–S电池中S还原的影响
Li-S电池是有前途的下一代电池技术。由于在电池操作过程中形成了可溶性多硫化物,所以电池的电解质组合物在指导可溶性多硫化锂的形成和形成中起着积极的作用。近来,由于它们相对于多硫化物的少量溶剂化性质,已经探索了新型的称为“溶剂化物”的电解质,其包含化学计量的盐和溶剂并在室温下形成液体。可以理解,溶剂化物电解质的粘度很高,限制了它们的生存能力。然而,可以引入对溶剂化物结构本身呈惰性的氢氟醚助溶剂,以降低粘度并增强扩散。纳扎尔(Nazar)和同事先前曾报道,添加1,1,2,2-四氟乙基2,2,3,2 – LiTFSI,与纯溶剂化物相比,可提高容量保留率。在这里,我们评估了TTE添加对Li–S电池的电化学行为和(MeCN)2 –LiTFSI电解质的溶剂化结构的影响。与先前的建议相反,拉曼光谱和NMR光谱结合从头算分子动力学模拟表明,TTE以Li +的形式与MeCN的配位关系相协调,从而在电解质中产生了较高含量的游离MeCN(一种良好的多硫化物溶剂)。游离MeCN含量较高的电解质可能会促进Li-S电池中S的电化学还原过程中更快的多硫化物形成动力学,这可能是由于游离MeCN的溶剂化作用所致。
更新日期:2016-12-08
中文翻译:

氢氟醚助溶剂的添加对乙腈基溶剂化物中Li溶剂化的影响及其对Li–S电池中S还原的影响
Li-S电池是有前途的下一代电池技术。由于在电池操作过程中形成了可溶性多硫化物,所以电池的电解质组合物在指导可溶性多硫化锂的形成和形成中起着积极的作用。近来,由于它们相对于多硫化物的少量溶剂化性质,已经探索了新型的称为“溶剂化物”的电解质,其包含化学计量的盐和溶剂并在室温下形成液体。可以理解,溶剂化物电解质的粘度很高,限制了它们的生存能力。然而,可以引入对溶剂化物结构本身呈惰性的氢氟醚助溶剂,以降低粘度并增强扩散。纳扎尔(Nazar)和同事先前曾报道,添加1,1,2,2-四氟乙基2,2,3,2 – LiTFSI,与纯溶剂化物相比,可提高容量保留率。在这里,我们评估了TTE添加对Li–S电池的电化学行为和(MeCN)2 –LiTFSI电解质的溶剂化结构的影响。与先前的建议相反,拉曼光谱和NMR光谱结合从头算分子动力学模拟表明,TTE以Li +的形式与MeCN的配位关系相协调,从而在电解质中产生了较高含量的游离MeCN(一种良好的多硫化物溶剂)。游离MeCN含量较高的电解质可能会促进Li-S电池中S的电化学还原过程中更快的多硫化物形成动力学,这可能是由于游离MeCN的溶剂化作用所致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号