当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Molecular Thermodynamic Model of Complexation in Mixtures of Oppositely Charged Polyelectrolytes with Explicit Account of Charge Association/Dissociation
Macromolecules ( IF 5.1 ) Pub Date : 2016-12-14 00:00:00 , DOI: 10.1021/acs.macromol.6b01464
Ali Salehi 1 , Ronald G. Larson 1
Macromolecules ( IF 5.1 ) Pub Date : 2016-12-14 00:00:00 , DOI: 10.1021/acs.macromol.6b01464
Ali Salehi 1 , Ronald G. Larson 1
Affiliation
|
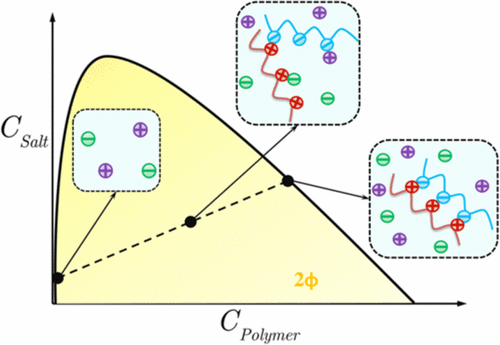
Into an extended Voorn–Overbeek (EVO) free energy model of polyelectrolyte (PE) complexation and phase behavior, we incorporate three classes of short-ranged electrostatic effects, namely counterion association–dissociation, cross-chain ion pairing (IP), and charge regulation by treating each as a reversible chemical reaction leading to a corresponding law of mass action in a self-consistent fashion. The importance of each reaction is controlled by a corresponding chemistry-dependent standard free energy input parameter. Our model also accounts for Born (or ion solvation) energy using a linear mixing rule for the effective dielectric constant. In monophasic systems, the proposed model can qualitatively explain the observed shifts in acidity and basicity observed in potentiometric titration of weak PEs in the presence of salt and oppositely charged PEs in accordance with Le Châtelier’s principle. We demonstrate how a competition between counterion condensation (CC) and IP alone can explain the complex coacervation of strongly charged PEs as well as the existence of a critical salt concentration. Binodal diagrams predicted in our model are also affected by long-ranged electrostatics and are most sensitive to IP strength both for weak and strong PEs. The extent of IP increases in the dense phase at the expense of reduced CC upon coacervation consistent with counter release view of complex coacervation. We compare binodal diagrams predicted by our model against experimental data for both weakly and strongly dissociating polyions pairs and find a plausible parameter set that leads to an acceptable and partial agreement with experiments in the two cases, respectively.
中文翻译:

带有电荷缔合/解离的显式解释的带相反电荷的聚电解质混合物中络合的分子热力学模型
将聚电解质(PE)络合和相行为的扩展的Voorn-Overbeek(EVO)自由能模型纳入三类短距离静电效应,即抗衡离子缔合-解离,跨链离子对(IP)和电荷通过将每个反应视为可逆的化学反应来调节,从而以自洽的方式产生相应的质量作用定律。每个反应的重要性由相应的化学依赖性标准自由能输入参数控制。我们的模型还使用线性混合法则来计算有效介电常数,从而计算出Born(或离子溶剂化)能。在单相系统中 根据LeChâtelier的原理,所提出的模型可以定性地解释在存在盐和带相反电荷的PE的情况下,电位滴定中弱PE的电位滴定中观察到的酸度和碱度变化。我们展示了抗衡离子缩合(CC)和IP单独竞争如何解释强电荷PE的复杂凝聚以及临界盐浓度的存在。在我们的模型中预测的双曲线图也受到远程静电的影响,无论是弱PE还是强PE都对IP强度最敏感。凝聚过程中IP的增加程度以致密化阶段CC减少的代价为代价,这与复杂凝聚过程的反释观点一致。
更新日期:2016-12-14
中文翻译:

带有电荷缔合/解离的显式解释的带相反电荷的聚电解质混合物中络合的分子热力学模型
将聚电解质(PE)络合和相行为的扩展的Voorn-Overbeek(EVO)自由能模型纳入三类短距离静电效应,即抗衡离子缔合-解离,跨链离子对(IP)和电荷通过将每个反应视为可逆的化学反应来调节,从而以自洽的方式产生相应的质量作用定律。每个反应的重要性由相应的化学依赖性标准自由能输入参数控制。我们的模型还使用线性混合法则来计算有效介电常数,从而计算出Born(或离子溶剂化)能。在单相系统中 根据LeChâtelier的原理,所提出的模型可以定性地解释在存在盐和带相反电荷的PE的情况下,电位滴定中弱PE的电位滴定中观察到的酸度和碱度变化。我们展示了抗衡离子缩合(CC)和IP单独竞争如何解释强电荷PE的复杂凝聚以及临界盐浓度的存在。在我们的模型中预测的双曲线图也受到远程静电的影响,无论是弱PE还是强PE都对IP强度最敏感。凝聚过程中IP的增加程度以致密化阶段CC减少的代价为代价,这与复杂凝聚过程的反释观点一致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号