当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical dynamics simulations of the monohydrated OH−(H2O) + CH3I reaction. Atomic-level mechanisms and comparison with experiment
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-06-25 14:20:07 , DOI: 10.1063/1.4922451
Jing Xie 1 , Rico Otto 2 , Roland Wester 3 , William L. Hase 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-06-25 14:20:07 , DOI: 10.1063/1.4922451
Jing Xie 1 , Rico Otto 2 , Roland Wester 3 , William L. Hase 1
Affiliation
|
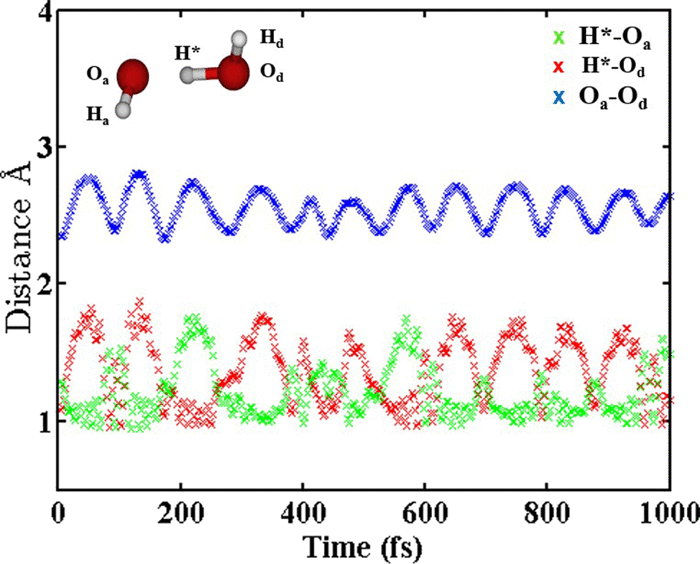
Direct dynamics simulations, with B97-1/ECP/d theory, were performed to study the role of microsolvation for the OH−(H2O) + CH3I reaction. The SN2 reaction dominates at all reactant collision energies, but at higher collision energies proton transfer to form CH2I−, and to a lesser extent CH2I− (H2O), becomes important. The SN2 reaction occurs by direct rebound and stripping mechanisms, and 28 different indirect atomistic mechanisms, with the latter dominating. Important components of the indirect mechanisms are the roundabout and formation of SN2 and proton transfer pre-reaction complexes and intermediates, including [CH3--I--OH]−. In contrast, for the unsolvated OH− + CH3I SN2 reaction, there are only seven indirect atomistic mechanisms and the direct mechanisms dominate. Overall, the simulation results for the OH−(H2O) + CH3IߙSN2 reaction are in good agreement with experiment with respect to reaction rate constant, product branching ratio, etc. Differences between simulation and experiment are present for the SN2 velocity scattering angle at high collision energies and the proton transfer probability at low collision energies. Equilibrium solvation by the H2O molecule is unimportant. The SN2 reaction is dominated by events in which H2O leaves the reactive system as CH3OH is formed or before CH3OH formation. Formation of solvated products is unimportant and participation of the (H2O)CH3OH---I− post-reaction complex for the SN2 reaction is negligible.
中文翻译:

一水合OH-(H2O)+ CH3I反应的化学动力学模拟。原子级机理及与实验的比较
直接动力学模拟,与B97-1 / ECP / d理论,进行研究microsolvation为OH的作用-(H 2 O)+ CH 3 I反应。在S Ñ 2反应占优势在所有反应物碰撞能量,但在更高的碰撞能量的质子转移,以形成CH 2我- ,并且在较小程度上CH 2我-(H 2 O),是重要的。S N 2反应通过直接反弹和剥离机制以及28种不同的间接原子机制发生,其中后者占主导地位。间接机制的重要组成部分是S N的回旋和形成2和质子转移反应前的配合物和中间体,包括[CH 3 -I-OH] -。相反,对于未溶剂化OH - + CH 3 I S Ñ 2反应,只有7主宰间接原子论机制和直接的机制。总体而言,模拟结果为OH -(H 2 O)+ CH 3我ߙ小号Ñ 2反应是在与实验一致相对于反应速率常数,产物分支比等仿真和实验之间的差异是存在用于S ñ高碰撞能量下的2个速度散射角和低碰撞能量下的质子转移概率。H 2 O分子的平衡溶剂化作用不重要。在S Ñ 2反应是通过其中H事件主导2 O的不足反应系统如CH 3 OH形成或之前CH 3 OH的形成。溶剂化的产品的形成是不重要和(H参与2 O)CH 3 OH ---我-后反应复杂用于S Ñ 2反应可以忽略不计。
更新日期:2015-06-26
中文翻译:

一水合OH-(H2O)+ CH3I反应的化学动力学模拟。原子级机理及与实验的比较
直接动力学模拟,与B97-1 / ECP / d理论,进行研究microsolvation为OH的作用-(H 2 O)+ CH 3 I反应。在S Ñ 2反应占优势在所有反应物碰撞能量,但在更高的碰撞能量的质子转移,以形成CH 2我- ,并且在较小程度上CH 2我-(H 2 O),是重要的。S N 2反应通过直接反弹和剥离机制以及28种不同的间接原子机制发生,其中后者占主导地位。间接机制的重要组成部分是S N的回旋和形成2和质子转移反应前的配合物和中间体,包括[CH 3 -I-OH] -。相反,对于未溶剂化OH - + CH 3 I S Ñ 2反应,只有7主宰间接原子论机制和直接的机制。总体而言,模拟结果为OH -(H 2 O)+ CH 3我ߙ小号Ñ 2反应是在与实验一致相对于反应速率常数,产物分支比等仿真和实验之间的差异是存在用于S ñ高碰撞能量下的2个速度散射角和低碰撞能量下的质子转移概率。H 2 O分子的平衡溶剂化作用不重要。在S Ñ 2反应是通过其中H事件主导2 O的不足反应系统如CH 3 OH形成或之前CH 3 OH的形成。溶剂化的产品的形成是不重要和(H参与2 O)CH 3 OH ---我-后反应复杂用于S Ñ 2反应可以忽略不计。

































 京公网安备 11010802027423号
京公网安备 11010802027423号