当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Molybdenum-Mediated Carbon Monoxide Deoxygenation and Coupling: Mono- and Dicarbyne Complexes Precede C–O Bond Cleavage and C–C Bond Formation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-12-12 , DOI: 10.1021/jacs.6b10535
Joshua A. Buss 1 , Theodor Agapie 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-12-12 , DOI: 10.1021/jacs.6b10535
Joshua A. Buss 1 , Theodor Agapie 1
Affiliation
|
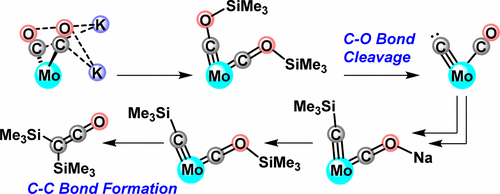
Deoxygenative coupling of CO to value-added C≥2 products is challenging and mechanistically poorly understood. Herein, we report a mechanistic investigation into the reductive coupling of CO, which provides new fundamental insights into a multielectron bond-breaking and bond-making transformation. In our studies, the formation of a bis(siloxycarbyne) complex precedes C-O bond cleavage. At -78 °C, over days, C-C coupling occurs without C-O cleavage. However, upon warming to 0 °C, C-O cleavage is observed from this bis(siloxycarbyne) complex. A siloxycarbyne/CO species undergoes C-O bond cleavage at lower temperatures, indicating that monosilylation, and a more electron-rich Mo center, favors deoxygenative pathways. From the bis(siloxycarbyne), isotopic labeling experiments and kinetics are consistent with a mechanism involving unimolecular silyl loss or C-O cleavage as rate-determining steps toward carbide formation. Reduction of Mo(IV) CO adducts of carbide and silylcarbyne species allowed for the spectroscopic detection of reduced silylcarbyne/CO and mixed silylcarbyne/siloxycarbyne complexes, respectively. Upon warming, both of these silylcarbynes undergo C-C bond formation, releasing silylated C2O1 fragments and demonstrating that the multiple bonded terminal Mo≡C moiety is an intermediate on the path to deoxygenated, C-C coupled products. The electronic structures of Mo carbide and carbyne species were investigated quantum mechanically. Overall, the present studies establish the elementary reactions steps by which CO is cleaved and coupled at a single metal site.
中文翻译:

钼介导的一氧化碳脱氧和偶联的机制:单碳炔配合物先于 C-O 键断裂和 C-C 键形成
CO 与增值 C≥2 产品的脱氧偶联具有挑战性,并且在机理上知之甚少。在此,我们报告了对 CO 还原耦合的机理研究,为多电子键断裂和成键转换提供了新的基本见解。在我们的研究中,双(甲硅烷氧基羰基)配合物的形成先于 CO 键断裂。在 -78 °C 下,几天后,CC 耦合发生而没有 CO 裂解。然而,当升温至 0 °C 时,可以从这种双(甲硅烷氧基羰基)复合物中观察到 CO 裂解。甲硅烷氧基羰基/CO 物种在较低温度下发生 CO 键断裂,表明单甲硅烷基化和更富电子的 Mo 中心有利于脱氧途径。从双(甲硅烷氧基羰基),同位素标记实验和动力学与涉及单分子甲硅烷基损失或 CO 裂解作为碳化物形成的速率决定步骤的机制一致。碳化物和甲硅烷基碳炔物种的 Mo(IV) CO 加合物的还原允许分别对还原的甲硅烷基碳炔/CO 和混合的甲硅烷基碳炔/甲硅烷氧基碳炔配合物进行光谱检测。加热后,这两种甲硅烷基羰基都形成 CC 键,释放出甲硅烷基化的 C2O1 片段,并证明多键合末端 Mo≡C 部分是脱氧、CC 偶联产物路径上的中间体。通过量子力学研究了碳化钼和碳炔物种的电子结构。总体而言,目前的研究建立了基本反应步骤,通过这些步骤,CO 在单个金属位点处被裂解和偶联。
更新日期:2016-12-12
中文翻译:

钼介导的一氧化碳脱氧和偶联的机制:单碳炔配合物先于 C-O 键断裂和 C-C 键形成
CO 与增值 C≥2 产品的脱氧偶联具有挑战性,并且在机理上知之甚少。在此,我们报告了对 CO 还原耦合的机理研究,为多电子键断裂和成键转换提供了新的基本见解。在我们的研究中,双(甲硅烷氧基羰基)配合物的形成先于 CO 键断裂。在 -78 °C 下,几天后,CC 耦合发生而没有 CO 裂解。然而,当升温至 0 °C 时,可以从这种双(甲硅烷氧基羰基)复合物中观察到 CO 裂解。甲硅烷氧基羰基/CO 物种在较低温度下发生 CO 键断裂,表明单甲硅烷基化和更富电子的 Mo 中心有利于脱氧途径。从双(甲硅烷氧基羰基),同位素标记实验和动力学与涉及单分子甲硅烷基损失或 CO 裂解作为碳化物形成的速率决定步骤的机制一致。碳化物和甲硅烷基碳炔物种的 Mo(IV) CO 加合物的还原允许分别对还原的甲硅烷基碳炔/CO 和混合的甲硅烷基碳炔/甲硅烷氧基碳炔配合物进行光谱检测。加热后,这两种甲硅烷基羰基都形成 CC 键,释放出甲硅烷基化的 C2O1 片段,并证明多键合末端 Mo≡C 部分是脱氧、CC 偶联产物路径上的中间体。通过量子力学研究了碳化钼和碳炔物种的电子结构。总体而言,目前的研究建立了基本反应步骤,通过这些步骤,CO 在单个金属位点处被裂解和偶联。

































 京公网安备 11010802027423号
京公网安备 11010802027423号