当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bond selectivity in electron-induced reaction due to directed recoil on an anisotropic substrate.
Nature Communications ( IF 14.7 ) Pub Date : 2016-12-09 , DOI: 10.1038/ncomms13690
Kelvin Anggara , Kai Huang , Lydie Leung , Avisek Chatterjee , Fang Cheng , John C. Polanyi
Nature Communications ( IF 14.7 ) Pub Date : 2016-12-09 , DOI: 10.1038/ncomms13690
Kelvin Anggara , Kai Huang , Lydie Leung , Avisek Chatterjee , Fang Cheng , John C. Polanyi
|
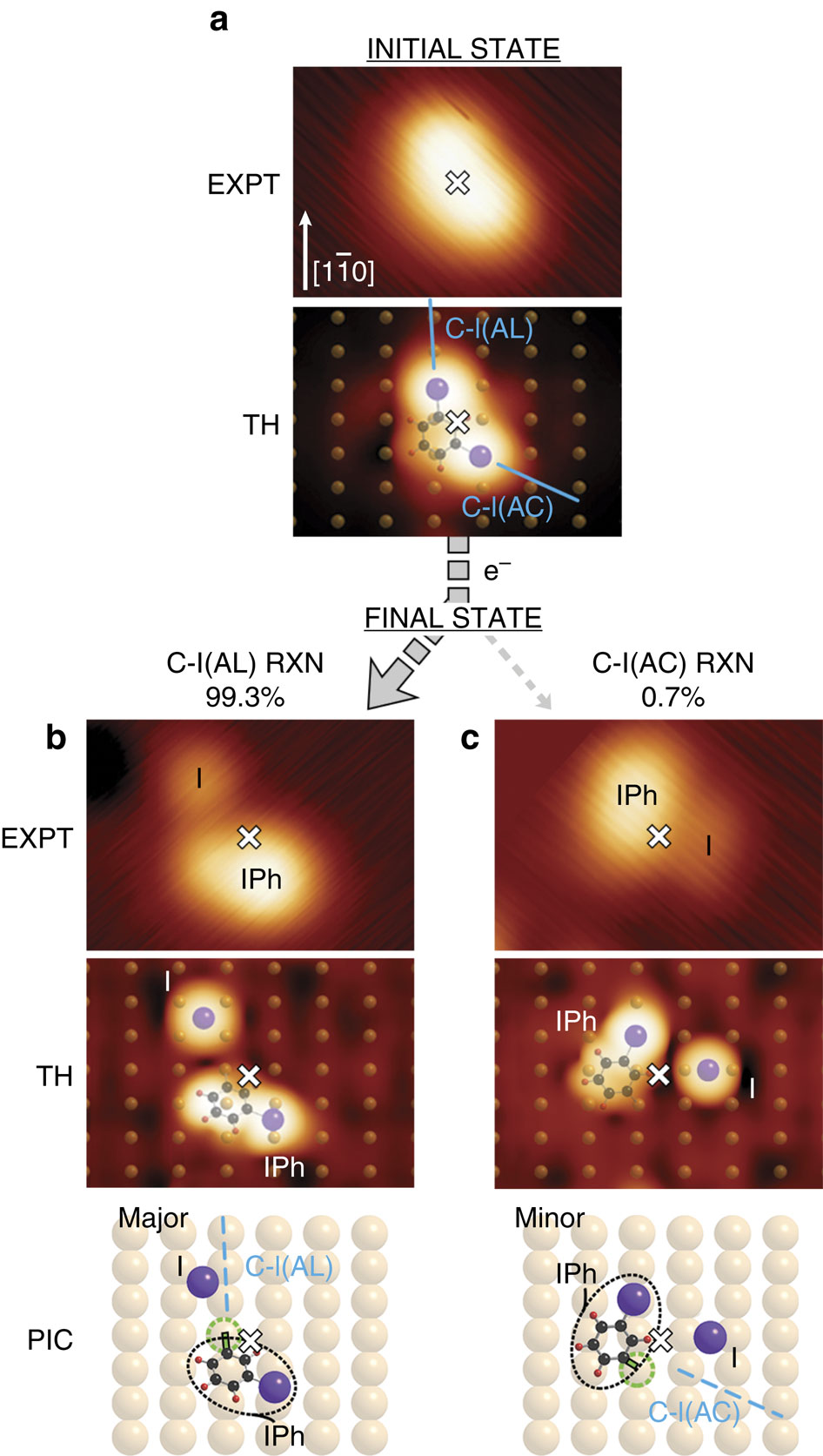
Bond-selective reaction is central to heterogeneous catalysis. In heterogeneous catalysis, selectivity is found to depend on the chemical nature and morphology of the substrate. Here, however, we show a high degree of bond selectivity dependent only on adsorbate bond alignment. The system studied is the electron-induced reaction of meta-diiodobenzene physisorbed on Cu(110). Of the adsorbate's C-I bonds, C-I aligned 'Along' the copper row dissociates in 99.3% of the cases giving surface reaction, whereas C-I bond aligned 'Across' the rows dissociates in only 0.7% of the cases. A two-electronic-state molecular dynamics model attributes reaction to an initial transition to a repulsive state of an Along C-I, followed by directed recoil of C towards a Cu atom of the same row, forming C-Cu. A similar impulse on an Across C-I gives directed C that, moving across rows, does not encounter a Cu atom and hence exhibits markedly less reaction.
中文翻译:

电子诱导反应中的键选择性是由于在各向异性基材上的定向反冲而引起的。
键选择反应是非均相催化的核心。在非均相催化中,发现选择性取决于底物的化学性质和形态。但是,在这里,我们显示出高度的键选择性仅取决于被吸附物键的排列。研究的系统是物理吸附在Cu(110)上的间二碘代苯的电子诱导反应。在被吸附物的CI键中,CI沿“ Along”排列的铜排在发生表面反应的情况下发生解离的比例为99.3%,而CI键沿“ Across”排列的排在仅0.7%的情况下发生离解。两电子态分子动力学模型将反应归因于沿CI的初始转变为排斥态,然后将C定向反冲到同一行的Cu原子,从而形成C-Cu。
更新日期:2016-12-12
中文翻译:

电子诱导反应中的键选择性是由于在各向异性基材上的定向反冲而引起的。
键选择反应是非均相催化的核心。在非均相催化中,发现选择性取决于底物的化学性质和形态。但是,在这里,我们显示出高度的键选择性仅取决于被吸附物键的排列。研究的系统是物理吸附在Cu(110)上的间二碘代苯的电子诱导反应。在被吸附物的CI键中,CI沿“ Along”排列的铜排在发生表面反应的情况下发生解离的比例为99.3%,而CI键沿“ Across”排列的排在仅0.7%的情况下发生离解。两电子态分子动力学模型将反应归因于沿CI的初始转变为排斥态,然后将C定向反冲到同一行的Cu原子,从而形成C-Cu。

































 京公网安备 11010802027423号
京公网安备 11010802027423号