当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A New Cross-Link for an Old Cross-Linking Drug: The Nitrogen Mustard Anticancer Agent Mechlorethamine Generates Cross-Links Derived from Abasic Sites in Addition to the Expected Drug-Bridged Cross-Links
Biochemistry ( IF 2.9 ) Pub Date : 2016-12-08 00:00:00 , DOI: 10.1021/acs.biochem.6b01080
Maryam Imani Nejad 1 , Kevin M. Johnson 1 , Nathan E. Price 1 , Kent S. Gates 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2016-12-08 00:00:00 , DOI: 10.1021/acs.biochem.6b01080
Maryam Imani Nejad 1 , Kevin M. Johnson 1 , Nathan E. Price 1 , Kent S. Gates 1, 2
Affiliation
|
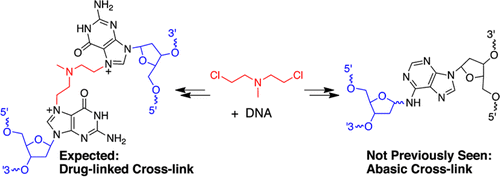
Nitrogen mustard anticancer drugs generate highly reactive aziridinium ions that alkylate DNA. Monoadducts arising from reaction with position N7 of guanine residues are the major DNA adducts generated by these agents. Interstrand cross-links in which the drug bridges position N7 of two guanine residues are formed in low yields relative to those of the monoadducts but are generally thought to be central to medicinal activity. The N7-alkylguanine residues generated by nitrogen mustards are depurinated to yield abasic (Ap) sites in duplex DNA. Here, we show that Ap sites generated by the nitrogen mustard mechlorethamine lead to interstrand cross-links of a type not previously associated with this drug. Gel electrophoretic data were consistent with early evolution of the expected drug-bridged cross-links, followed by the appearance of Ap-derived cross-links. The evidence is further consistent with a reaction pathway involving alkylation of a guanine residue in a 5′-GT sequence, followed by depurination to generate the Ap site, and cross-link formation via reaction of the Ap aldehyde residue with the opposing adenine residue at this site [Price, N. E., Johnson, K. M., Wang, J., Fekry, M. I., Wang, Y., and Gates, K. S. (2014) J. Am. Chem. Soc. 136, 3483–3490]. The monofunctional DNA-alkylating agents 2-chloro-N,N-diethylethanamine 5, (2-chloroethyl)ethylsulfide 6, and natural product leinamycin similarly were found to induce the formation of Ap-derived cross-links in duplex DNA. This work provides the first characterization of Ap-derived cross-links at sequences in which a cytosine residue is located directly opposing the Ap site. Cross-linking processes of this type could be relevant in medicine and biology because Ap sites with directly opposing cytosine residues occur frequently in genomic DNA via spontaneous or enzymatic depurination of guanine and N7-alkylguanine residues.
中文翻译:

一种旧的交叉链接药物的新的交叉链接:氮芥末抗癌剂甲氯乙胺除了预期的药物桥连的交叉链接之外,还产生了从无碱性位点衍生出来的交叉链接。
氮芥末抗癌药会产生高反应性的氮丙啶鎓离子,从而使DNA烷基化。与鸟嘌呤残基的N7位反应所产生的单加合物是这些试剂产生的主要DNA加合物。相对于单加合物,链间交联形成的药物跨两个鸟嘌呤残基的位置N7的产率相对于一加合物的产率低,但通常被认为是药物活性的中心。将氮芥产生的N7-烷基鸟嘌呤残基脱嘌呤以在双链DNA中产生无碱基(Ap)位点。在这里,我们显示了由氮芥芥甲氧乙胺产生的Ap位点导致以前与该药物不相关的链间交联。凝胶电泳数据与预期的药物桥联交联的早期演变相一致,其次是Ap衍生的交联的出现。该证据进一步与反应途径有关,该反应途径涉及将5'-GT序列中的鸟嘌呤残基烷基化,然后进行纯化以生成Ap位点,并通过Ap醛基残基与相对的腺嘌呤残基在16位的反应而形成交联。这个网站[Price,NE,Johnson,KM,Wang,J.,Fekry,MI,Wang,Y.,and Gates,KS(2014)J.上午 化学 Soc。136,3483-3490]。类似地,发现单官能DNA烷基化剂2-氯-N,N-二乙基乙胺5,(2-氯乙基)乙基硫化物6和天然产物莱那霉素可诱导双链DNA中Ap衍生的交联的形成。这项工作提供了在胞嘧啶残基位于与Ap位点直接相对的序列上Ap衍生的交联的第一个特征。这种类型的交联过程可能与医学和生物学有关,因为具有直接相反的胞嘧啶残基的Ap位点经常通过鸟嘌呤和N7-烷基鸟嘌呤残基的自发或酶促脱嘌呤出现在基因组DNA中。
更新日期:2016-12-08
中文翻译:

一种旧的交叉链接药物的新的交叉链接:氮芥末抗癌剂甲氯乙胺除了预期的药物桥连的交叉链接之外,还产生了从无碱性位点衍生出来的交叉链接。
氮芥末抗癌药会产生高反应性的氮丙啶鎓离子,从而使DNA烷基化。与鸟嘌呤残基的N7位反应所产生的单加合物是这些试剂产生的主要DNA加合物。相对于单加合物,链间交联形成的药物跨两个鸟嘌呤残基的位置N7的产率相对于一加合物的产率低,但通常被认为是药物活性的中心。将氮芥产生的N7-烷基鸟嘌呤残基脱嘌呤以在双链DNA中产生无碱基(Ap)位点。在这里,我们显示了由氮芥芥甲氧乙胺产生的Ap位点导致以前与该药物不相关的链间交联。凝胶电泳数据与预期的药物桥联交联的早期演变相一致,其次是Ap衍生的交联的出现。该证据进一步与反应途径有关,该反应途径涉及将5'-GT序列中的鸟嘌呤残基烷基化,然后进行纯化以生成Ap位点,并通过Ap醛基残基与相对的腺嘌呤残基在16位的反应而形成交联。这个网站[Price,NE,Johnson,KM,Wang,J.,Fekry,MI,Wang,Y.,and Gates,KS(2014)J.上午 化学 Soc。136,3483-3490]。类似地,发现单官能DNA烷基化剂2-氯-N,N-二乙基乙胺5,(2-氯乙基)乙基硫化物6和天然产物莱那霉素可诱导双链DNA中Ap衍生的交联的形成。这项工作提供了在胞嘧啶残基位于与Ap位点直接相对的序列上Ap衍生的交联的第一个特征。这种类型的交联过程可能与医学和生物学有关,因为具有直接相反的胞嘧啶残基的Ap位点经常通过鸟嘌呤和N7-烷基鸟嘌呤残基的自发或酶促脱嘌呤出现在基因组DNA中。

































 京公网安备 11010802027423号
京公网安备 11010802027423号