当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aryl Ketone Catalyzed Radical Allylation of C(sp3)–H Bonds under Photoirradiation
Organic Letters ( IF 4.9 ) Pub Date : 2016-12-08 00:00:00 , DOI: 10.1021/acs.orglett.6b03586 Shin Kamijo 1 , Kaori Kamijo 1 , Kiyotaka Maruoka 1 , Toshihiro Murafuji 1
Organic Letters ( IF 4.9 ) Pub Date : 2016-12-08 00:00:00 , DOI: 10.1021/acs.orglett.6b03586 Shin Kamijo 1 , Kaori Kamijo 1 , Kiyotaka Maruoka 1 , Toshihiro Murafuji 1
Affiliation
|
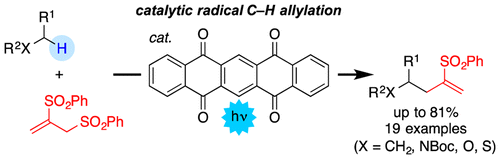
The catalytic introduction of an allyl group at nonacidic C(sp3)–H bonds was achieved under photoirradiation, in which 1,2-bis(phenylsulfonyl)-2-propene acts as an allyl source and 5,7,12,14-pentacenetetrone (PT) works as a C–H bond-cleaving catalyst. A variety of substances, including alkanes, carbamates, ethers, sulfides, and alcohols, were chemoselectively allylated in a single step under neutral conditions. The present transformation is catalyzed solely by an organic molecule, PT, and proceeds smoothly even under visible light irradiation (425 nm) in the case of alkanes as a starting substance.
中文翻译:

芳基酮在光辐照下催化C(sp 3)–H键的自由基烯丙基化
在光辐照下实现了在非酸性C(sp 3)-H键处烯丙基的催化引入,其中1,2-双(苯磺酰基)-2-丙烯为烯丙基源,5,7,12,14- pentacenetetrone(PT)可作为C–H键裂解催化剂。在中性条件下,只需一步即可将烷烃,氨基甲酸酯,醚,硫化物和醇等多种物质化学选择性烯丙基化。本转化仅由有机分子PT催化,并且在以烷烃为起始物质的情况下,即使在可见光照射(425nm)下也能顺利进行。
更新日期:2016-12-08
中文翻译:

芳基酮在光辐照下催化C(sp 3)–H键的自由基烯丙基化
在光辐照下实现了在非酸性C(sp 3)-H键处烯丙基的催化引入,其中1,2-双(苯磺酰基)-2-丙烯为烯丙基源,5,7,12,14- pentacenetetrone(PT)可作为C–H键裂解催化剂。在中性条件下,只需一步即可将烷烃,氨基甲酸酯,醚,硫化物和醇等多种物质化学选择性烯丙基化。本转化仅由有机分子PT催化,并且在以烷烃为起始物质的情况下,即使在可见光照射(425nm)下也能顺利进行。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号