当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and spectroscopic studies of highly fluorescent, solvatochromic diastereomers with differentially stacked bithiophene-substituted quinoxaline rings
Tetrahedron ( IF 2.1 ) Pub Date : 2016-12-08 08:28:11 Ryan A. Ciufo, John J. Kreinbihl, Sarah R. Johnson, Jocelyn M. Nadeau
Tetrahedron ( IF 2.1 ) Pub Date : 2016-12-08 08:28:11 Ryan A. Ciufo, John J. Kreinbihl, Sarah R. Johnson, Jocelyn M. Nadeau
|
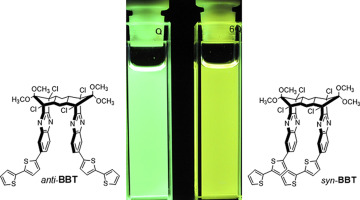
Diastereomeric C-shaped molecules containing closely stacked bithiophene-substituted quinoxaline rings were synthesized and characterized by NMR, UV–vis absorption, and fluorescence spectroscopy. The unique geometry of each diastereomer resulted in different degrees of π-overlap between the bithiophene-substituted quinoxaline ring chromophores, modulating their spectroscopic properties. The donor-acceptor nature of this chromophore gave rise to its positive solvatochromism. 1H NMR and UV–vis absorption spectroscopy confirmed the existence of π-π interactions in the ground state between the quinoxaline rings in both molecules but between the bithiophene rings only in the syn isomer. They exhibited significant emission maxima bathochromic shifts, a strong, positive solvatochromism, increased band broadening, and larger Stokes shifts when compared to a compound with an unstacked chromophore. Additionally, the syn isomer consistently showed λ max,em value red-shifts and larger band broadening and Stokes shifts compared to the anti isomer due to the greater π-overlap in the syn isomer.
中文翻译:

具有差异堆积的联噻吩取代的喹喔啉环的高荧光,溶剂变色非对映异构体的合成和光谱研究
合成了包含紧密堆积的联噻吩取代的喹喔啉环的非对映体C形分子,并通过NMR,UV-vis吸收和荧光光谱进行了表征。每个非对映异构体的独特几何结构导致联噻吩取代的喹喔啉环生色团之间的π重叠程度不同,从而调节了它们的光谱性质。该生色团的供体-受体性质导致其正溶剂化变色。1个1 H NMR和UV-vis吸收光谱证实了在两个分子中的喹喔啉环之间,而仅在同分异构体的联噻吩环之间的基态中存在π-π相互作用。与未堆叠发色团的化合物相比,它们表现出显着的最大发射红移,强烈的正溶剂变色,增加的谱带展宽和更大的斯托克斯频移。另外,由于反式异构体的π重叠较大,所以与反式异构体相比,同式异构体始终显示出λmax,em值红移和较大的谱带展宽和斯托克斯频移。
更新日期:2016-12-09
中文翻译:

具有差异堆积的联噻吩取代的喹喔啉环的高荧光,溶剂变色非对映异构体的合成和光谱研究
合成了包含紧密堆积的联噻吩取代的喹喔啉环的非对映体C形分子,并通过NMR,UV-vis吸收和荧光光谱进行了表征。每个非对映异构体的独特几何结构导致联噻吩取代的喹喔啉环生色团之间的π重叠程度不同,从而调节了它们的光谱性质。该生色团的供体-受体性质导致其正溶剂化变色。1个1 H NMR和UV-vis吸收光谱证实了在两个分子中的喹喔啉环之间,而仅在同分异构体的联噻吩环之间的基态中存在π-π相互作用。与未堆叠发色团的化合物相比,它们表现出显着的最大发射红移,强烈的正溶剂变色,增加的谱带展宽和更大的斯托克斯频移。另外,由于反式异构体的π重叠较大,所以与反式异构体相比,同式异构体始终显示出λmax,em值红移和较大的谱带展宽和斯托克斯频移。






























 京公网安备 11010802027423号
京公网安备 11010802027423号