当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfination of Alcohols with p‐Toluenesulfonylmethyl Isocyanide under Metal‐Free Conditions: A Mitsunobu Approach
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-11-25 , DOI: 10.1002/adsc.201600997 Lingaswamy Kadari 1 , Palakodety Radha Krishna 1 , Y. Lakshmi Prapurna 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-11-25 , DOI: 10.1002/adsc.201600997 Lingaswamy Kadari 1 , Palakodety Radha Krishna 1 , Y. Lakshmi Prapurna 1
Affiliation
|
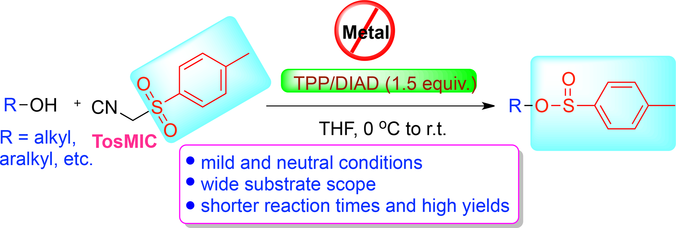
A Mitsunobu approach for the synthesis of sulfinate esters by direct nucleophilic substitution of alcohols is described. The salient features of this strategy include neutral and metal‐free conditions for the rapid synthesis of sulfinates in high yields. The present protocol using p‐toluenesulfonylmethyl isocyanide (TosMIC) and the triphenylphosphine (TPP)/diisopropyl azodicarboxylate (DIAD) reagent system represents the general synthetic route to this important class of compounds.
中文翻译:

在无金属条件下用对甲苯磺酰基甲基异氰酸酯将酒精硫化:Mitsunobu方法
描述了通过醇的直接亲核取代来合成亚磺酸酯的Mitsunobu方法。该策略的显着特征包括中性和无金属条件,可高产率快速合成亚磺酸盐。使用对甲苯磺酰基甲基异氰化物(TosMIC)和三苯基膦(TPP)/偶氮二异丙基二异丙酯(DIAD)试剂系统的本方案代表了这一重要化合物类别的一般合成路线。
更新日期:2016-11-25
中文翻译:

在无金属条件下用对甲苯磺酰基甲基异氰酸酯将酒精硫化:Mitsunobu方法
描述了通过醇的直接亲核取代来合成亚磺酸酯的Mitsunobu方法。该策略的显着特征包括中性和无金属条件,可高产率快速合成亚磺酸盐。使用对甲苯磺酰基甲基异氰化物(TosMIC)和三苯基膦(TPP)/偶氮二异丙基二异丙酯(DIAD)试剂系统的本方案代表了这一重要化合物类别的一般合成路线。































 京公网安备 11010802027423号
京公网安备 11010802027423号