当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orbital Control of Photochemical Rearrangement of 4-Aryl-1,1-dicyano-1-butenes through the Hyperconjugative Substitution on the Linker Chain
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2016-11-22 00:00:00 , DOI: 10.1021/acs.jpclett.6b02632
Nobuo Matsuki 1 , Yoshihisa Inoue 1 , Tadashi Mori 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2016-11-22 00:00:00 , DOI: 10.1021/acs.jpclett.6b02632
Nobuo Matsuki 1 , Yoshihisa Inoue 1 , Tadashi Mori 1
Affiliation
|
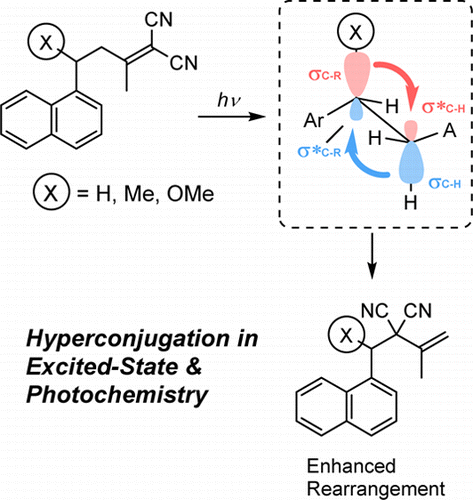
Hyperconjugative interaction was demonstrated to play a vital role in the photochemistry of 4-aryl-1,1-dicyano-1-butenes. Thus a simple substituent on the benzylic position effectively induced a new photoreactivity to afford an allylic rearrangement product that is not obtained for the parent substrate. The natural bond orbital analysis was employed to reveal the enhanced relative contributions of hyperconjugation in the excited state, which dramatically alter the photochemical outcomes not only by reducing the strength of the allylic/benzylic bond but more crucially by affecting the conformer distribution.
中文翻译:

通过连接子链上的高共轭取代作用对4-芳基-1,1-二氰基-1-丁烯进行光化学重排的轨道控制
高共轭相互作用被证明在4-芳基-1,1-二氰基-1-丁烯的光化学中起着至关重要的作用。因此,苄基位置上的简单取代基有效地诱导了新的光反应性,从而提供了母体底物无法获得的烯丙基重排产物。利用自然键轨道分析揭示了在激发态下超共轭反应的相对贡献增加,这不仅显着改变了烯丙基/苄基键的强度,而且更重要地是通过影响构象异构体的分布,极大地改变了光化学结果。
更新日期:2016-11-22
中文翻译:

通过连接子链上的高共轭取代作用对4-芳基-1,1-二氰基-1-丁烯进行光化学重排的轨道控制
高共轭相互作用被证明在4-芳基-1,1-二氰基-1-丁烯的光化学中起着至关重要的作用。因此,苄基位置上的简单取代基有效地诱导了新的光反应性,从而提供了母体底物无法获得的烯丙基重排产物。利用自然键轨道分析揭示了在激发态下超共轭反应的相对贡献增加,这不仅显着改变了烯丙基/苄基键的强度,而且更重要地是通过影响构象异构体的分布,极大地改变了光化学结果。



































 京公网安备 11010802027423号
京公网安备 11010802027423号