当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of spontaneous filament nucleation via oligomers: Insights from theory and simulation
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-11-22 16:00:23 , DOI: 10.1063/1.4965040 Anđela Šarić 1, 2 , Thomas C. T. Michaels 2, 3 , Alessio Zaccone 4 , Tuomas P. J. Knowles 2 , Daan Frenkel 2
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2016-11-22 16:00:23 , DOI: 10.1063/1.4965040 Anđela Šarić 1, 2 , Thomas C. T. Michaels 2, 3 , Alessio Zaccone 4 , Tuomas P. J. Knowles 2 , Daan Frenkel 2
Affiliation
|
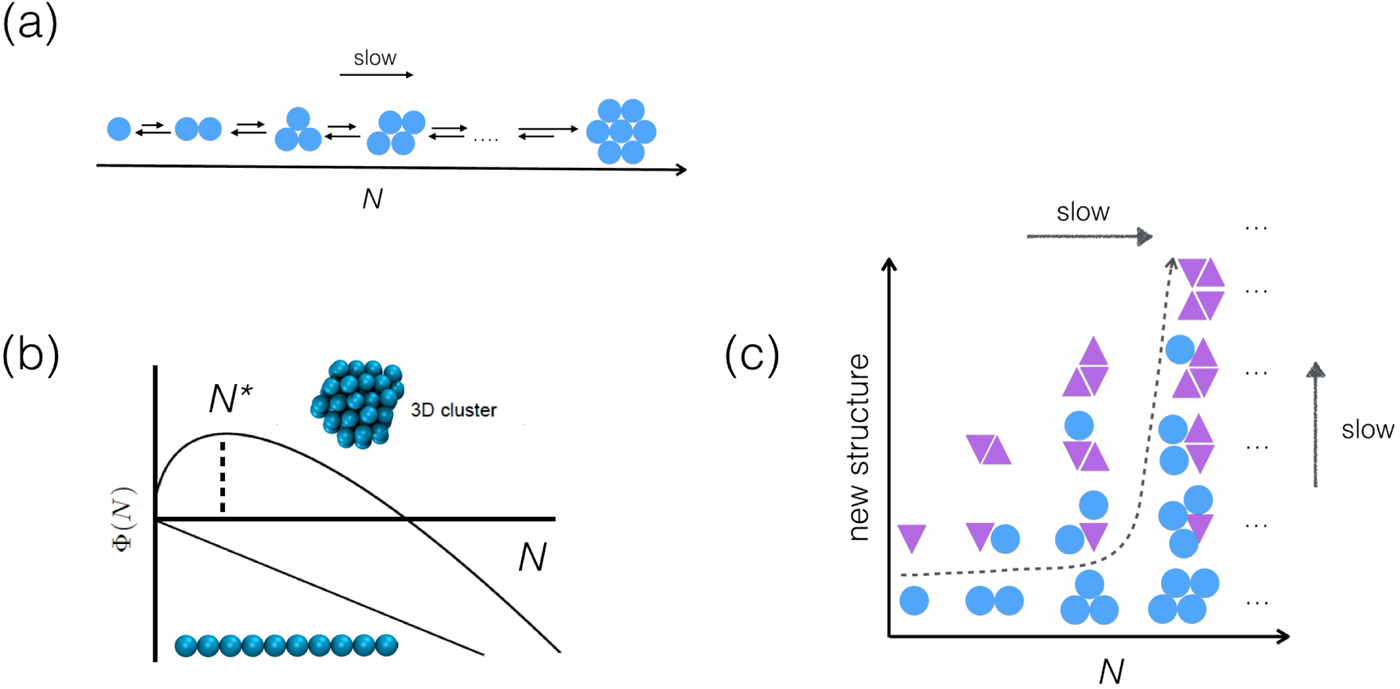
Nucleation processes are at the heart of a large number of phenomena, from cloud formation to protein crystallization. A recently emerging area where nucleation is highly relevant is the initiation of filamentous protein self-assembly, a process that has broad implications in many research areas ranging from medicine to nanotechnology. As such, spontaneous nucleation of protein fibrils has received much attention in recent years with many theoretical and experimental studies focussing on the underlying physical principles. In this paper we make a step forward in this direction and explore the early time behaviour of filamentous protein growth in the context of nucleation theory. We first provide an overview of the thermodynamics and kinetics of spontaneous nucleation of protein filaments in the presence of one relevant degree of freedom, namely the cluster size. In this case, we review how key kinetic observables, such as the reaction order of spontaneous nucleation, are directly related to the physical size of the critical nucleus. We then focus on the increasingly prominent case of filament nucleation that includes a conformational conversion of the nucleating building-block as an additional slow step in the nucleation process. Using computer simulations, we study the concentration dependence of the nucleation rate. We find that, under these circumstances, the reaction order of spontaneous nucleation with respect to the free monomer does no longer relate to the overall physical size of the nucleating aggregate but rather to the portion of the aggregate that actively participates in the conformational conversion. Our results thus provide a novel interpretation of the common kinetic descriptors of protein filament formation, including the reaction order of the nucleation step or the scaling exponent of lag times, and put into perspective current theoretical descriptions of protein aggregation.
中文翻译:

通过低聚物自发的长丝成核动力学:理论和模拟的见解
从云形成到蛋白质结晶,成核过程是许多现象的核心。近年来,与成核高度相关的新兴领域是丝状蛋白质自组装的开始,这一过程在从医学到纳米技术的许多研究领域中具有广泛的意义。因此,近年来,蛋白原纤维的自发成核受到了广泛的关注,许多理论和实验研究都集中在基本的物理原理上。在本文中,我们朝着这个方向迈出了一步,并在成核理论的背景下探索了丝状蛋白生长的早期行为。我们首先概述在存在一个相关自由度的情况下蛋白丝自发成核的热力学和动力学,即集群大小。在这种情况下,我们回顾了关键的动力学观测值(例如自发成核的反应顺序)如何与关键核的物理尺寸直接相关。然后,我们关注于长丝成核的日益突出的案例,其中包括成核构件的构象转换,这是成核过程中另一个缓慢的步骤。使用计算机模拟,我们研究成核速率的浓度依赖性。我们发现,在这些情况下,相对于游离单体的自发成核反应的顺序不再与成核聚集体的整体物理尺寸有关,而是与有效参与构象转化的聚集体部分有关。
更新日期:2016-11-23
中文翻译:

通过低聚物自发的长丝成核动力学:理论和模拟的见解
从云形成到蛋白质结晶,成核过程是许多现象的核心。近年来,与成核高度相关的新兴领域是丝状蛋白质自组装的开始,这一过程在从医学到纳米技术的许多研究领域中具有广泛的意义。因此,近年来,蛋白原纤维的自发成核受到了广泛的关注,许多理论和实验研究都集中在基本的物理原理上。在本文中,我们朝着这个方向迈出了一步,并在成核理论的背景下探索了丝状蛋白生长的早期行为。我们首先概述在存在一个相关自由度的情况下蛋白丝自发成核的热力学和动力学,即集群大小。在这种情况下,我们回顾了关键的动力学观测值(例如自发成核的反应顺序)如何与关键核的物理尺寸直接相关。然后,我们关注于长丝成核的日益突出的案例,其中包括成核构件的构象转换,这是成核过程中另一个缓慢的步骤。使用计算机模拟,我们研究成核速率的浓度依赖性。我们发现,在这些情况下,相对于游离单体的自发成核反应的顺序不再与成核聚集体的整体物理尺寸有关,而是与有效参与构象转化的聚集体部分有关。

































 京公网安备 11010802027423号
京公网安备 11010802027423号