当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced potency of bivalent small molecule gp41 inhibitors
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2016-11-30 11:06:10
Vladimir Sofiyev, Hardeep Kaur, Beth A. Snyder, Priscilla A. Hogan, Roger G. Ptak, Peter Hwang, Miriam Gochin
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2016-11-30 11:06:10
Vladimir Sofiyev, Hardeep Kaur, Beth A. Snyder, Priscilla A. Hogan, Roger G. Ptak, Peter Hwang, Miriam Gochin
|
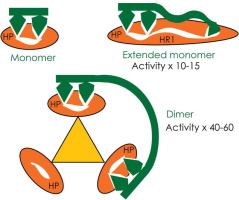
Low molecular weight peptidomimetic inhibitors with hydrophobic pocket binding properties and moderate fusion inhibitory activity against HIV-1 gp41-mediated cell fusion were elaborated by increasing the available surface area for interacting with the heptad repeat-1 (HR1) coiled coil on gp41. Two types of modifications were tested: 1) increasing the overall hydrophobicity of the molecules with an extension that could interact in the HR1 groove, and 2) forming symmetrical dimers with two peptidomimetic motifs that could potentially interact simultaneously in two hydrophobic pockets on the HR1 trimer. The latter approach was more successful, yielding 40–60times improved potency against HIV fusion over the monomers. Biophysical characterization, including equilibrium binding studies by fluorescence and kinetic analysis by Surface Plasmon Resonance, revealed that inhibitor potency was better correlated to off-rates than to binding affinity. Binding and kinetic data could be fit to a model of bidentate interaction of dimers with the HR1 trimer as an explanation for the slow off-rate, albeit with minimal cooperativity due to the highly flexible ligand structures. The strong cooperativity observed in fusion inhibitory activity of the dimers implied accentuated potency due to the transient nature of the targeted intermediate. Optimization of monomer, dimer or higher order structures has the potential to lead to highly potent non-peptide fusion inhibitors by targeting multiple hydrophobic pockets.
中文翻译:

二价小分子gp41抑制剂的效能增强
通过增加可利用的表面积来与gp41上的七肽重复序列1(HR1)盘绕的线圈相互作用,来设计具有疏水性口袋结合特性和对HIV-1 gp41介导的细胞融合具有中等融合抑制活性的低分子量拟肽抑制剂。测试了两种类型的修饰:1)通过延伸可以在HR1凹槽中相互作用的延伸来增加分子的整体疏水性,以及2)形成具有两个拟肽基序的对称二聚体,它们可能同时在HR1三聚体的两个疏水性口袋中相互作用。后一种方法更成功,与单体相比,抗HIV融合的效力提高了40-60倍。生物物理表征,包括通过荧光进行的平衡结合研究和通过表面等离振子共振进行的动力学分析,揭示抑制剂的效力与解离速率的相关性比与结合亲和力的相关性更好。结合和动力学数据可能适合于二聚体与HR1三聚体的二齿相互作用模型,以解释缓慢的脱模速率,尽管由于高度灵活的配体结构而具有最小的协同性。由于目标中间体的瞬时性质,在二聚体的融合抑制活性中观察到的强合作性暗示了增强的效力。通过靶向多个疏水口袋,单体,二聚体或更高阶结构的优化可能导致产生高效的非肽融合抑制剂。结合和动力学数据可能适合于二聚体与HR1三聚体的二齿相互作用模型,以解释缓慢的脱模速率,尽管由于高度灵活的配体结构而具有最小的协同性。由于目标中间体的瞬时性质,在二聚体的融合抑制活性中观察到的强合作性暗示了增强的效力。通过靶向多个疏水口袋,单体,二聚体或更高阶结构的优化可能导致产生高效的非肽融合抑制剂。结合和动力学数据可能适合于二聚体与HR1三聚体的二齿相互作用模型,以解释缓慢的脱模速率,尽管由于高度灵活的配体结构而具有最小的协同性。由于目标中间体的瞬时性质,在二聚体的融合抑制活性中观察到的强合作性暗示了增强的效力。通过靶向多个疏水口袋,单体,二聚体或更高阶结构的优化可能导致产生高效的非肽融合抑制剂。
更新日期:2016-12-01
中文翻译:

二价小分子gp41抑制剂的效能增强
通过增加可利用的表面积来与gp41上的七肽重复序列1(HR1)盘绕的线圈相互作用,来设计具有疏水性口袋结合特性和对HIV-1 gp41介导的细胞融合具有中等融合抑制活性的低分子量拟肽抑制剂。测试了两种类型的修饰:1)通过延伸可以在HR1凹槽中相互作用的延伸来增加分子的整体疏水性,以及2)形成具有两个拟肽基序的对称二聚体,它们可能同时在HR1三聚体的两个疏水性口袋中相互作用。后一种方法更成功,与单体相比,抗HIV融合的效力提高了40-60倍。生物物理表征,包括通过荧光进行的平衡结合研究和通过表面等离振子共振进行的动力学分析,揭示抑制剂的效力与解离速率的相关性比与结合亲和力的相关性更好。结合和动力学数据可能适合于二聚体与HR1三聚体的二齿相互作用模型,以解释缓慢的脱模速率,尽管由于高度灵活的配体结构而具有最小的协同性。由于目标中间体的瞬时性质,在二聚体的融合抑制活性中观察到的强合作性暗示了增强的效力。通过靶向多个疏水口袋,单体,二聚体或更高阶结构的优化可能导致产生高效的非肽融合抑制剂。结合和动力学数据可能适合于二聚体与HR1三聚体的二齿相互作用模型,以解释缓慢的脱模速率,尽管由于高度灵活的配体结构而具有最小的协同性。由于目标中间体的瞬时性质,在二聚体的融合抑制活性中观察到的强合作性暗示了增强的效力。通过靶向多个疏水口袋,单体,二聚体或更高阶结构的优化可能导致产生高效的非肽融合抑制剂。结合和动力学数据可能适合于二聚体与HR1三聚体的二齿相互作用模型,以解释缓慢的脱模速率,尽管由于高度灵活的配体结构而具有最小的协同性。由于目标中间体的瞬时性质,在二聚体的融合抑制活性中观察到的强合作性暗示了增强的效力。通过靶向多个疏水口袋,单体,二聚体或更高阶结构的优化可能导致产生高效的非肽融合抑制剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号