当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An elastic element in the protocadherin-15 tip link of the inner ear.
Nature Communications ( IF 14.7 ) Pub Date : 2016-11-18 , DOI: 10.1038/ncomms13458 Raul Araya-Secchi 1 , Brandon L Neel 1 , Marcos Sotomayor 1
Nature Communications ( IF 14.7 ) Pub Date : 2016-11-18 , DOI: 10.1038/ncomms13458 Raul Araya-Secchi 1 , Brandon L Neel 1 , Marcos Sotomayor 1
Affiliation
|
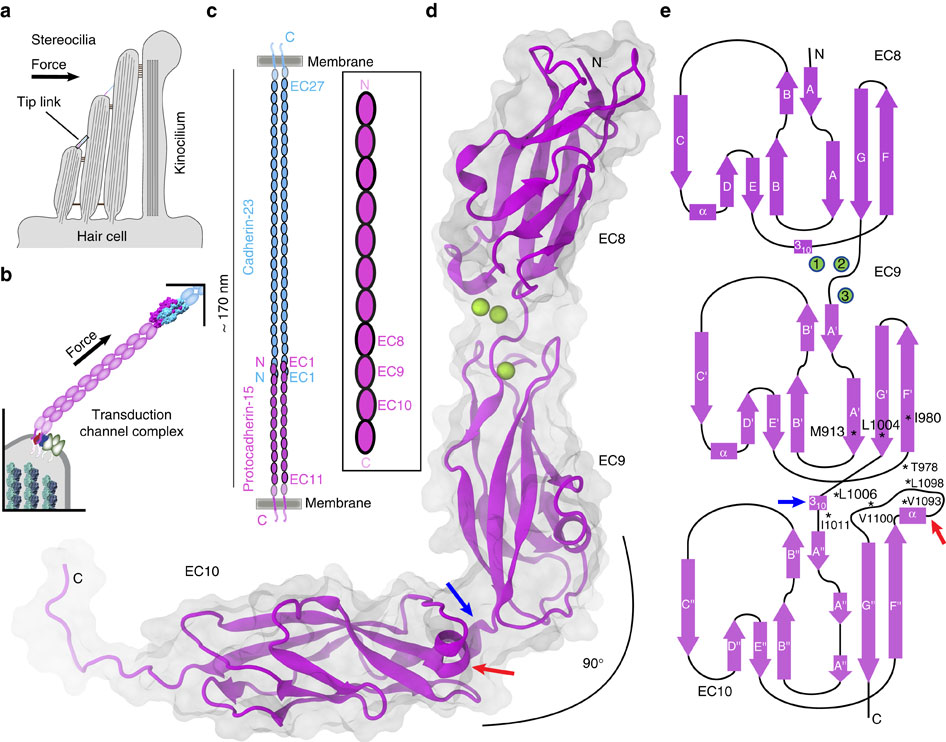
Tip link filaments convey force and gate inner-ear hair-cell transduction channels to mediate perception of sound and head movements. Cadherin-23 and protocadherin-15 form tip links through a calcium-dependent interaction of their extracellular domains made of multiple extracellular cadherin (EC) repeats. These repeats are structurally similar, but not identical in sequence, often featuring linkers with conserved calcium-binding sites that confer mechanical strength to them. Here we present the X-ray crystal structures of human protocadherin-15 EC8-EC10 and mouse EC9-EC10, which show an EC8-9 canonical-like calcium-binding linker, and an EC9-10 calcium-free linker that alters the linear arrangement of EC repeats. Molecular dynamics simulations and small-angle X-ray scattering experiments support this non-linear conformation. Simulations also suggest that unbending of EC9-10 confers some elasticity to otherwise rigid tip links. The new structure provides a first view of protocadherin-15's non-canonical EC linkers and suggests how they may function in inner-ear mechanotransduction, with implications for other cadherins.
中文翻译:

内耳原钙粘蛋白 15 尖端连接中的弹性元件。
尖端连接细丝传递力并控制内耳毛细胞转导通道,以介导声音和头部运动的感知。 Cadherin-23 和原钙粘蛋白-15 通过由多个细胞外钙粘蛋白 (EC) 重复组成的细胞外结构域的钙依赖性相互作用形成尖端连接。这些重复序列在结构上相似,但序列不相同,通常具有具有保守的钙结合位点的接头,从而赋予它们机械强度。在这里,我们展示了人原钙粘蛋白-15 EC8-EC10 和小鼠 EC9-EC10 的 X 射线晶体结构,其中显示了 EC8-9 典型的钙结合接头,以及改变线性的 EC9-10 无钙接头。 EC重复序列的排列。分子动力学模拟和小角X射线散射实验支持这种非线性构象。模拟还表明,EC9-10 的不弯曲赋予了刚性尖端连杆一定的弹性。新结构提供了原钙粘蛋白 15 的非典型 EC 连接子的第一视角,并表明它们如何在内耳机械转导中发挥作用,这对其他钙粘蛋白具有影响。
更新日期:2016-11-20
中文翻译:

内耳原钙粘蛋白 15 尖端连接中的弹性元件。
尖端连接细丝传递力并控制内耳毛细胞转导通道,以介导声音和头部运动的感知。 Cadherin-23 和原钙粘蛋白-15 通过由多个细胞外钙粘蛋白 (EC) 重复组成的细胞外结构域的钙依赖性相互作用形成尖端连接。这些重复序列在结构上相似,但序列不相同,通常具有具有保守的钙结合位点的接头,从而赋予它们机械强度。在这里,我们展示了人原钙粘蛋白-15 EC8-EC10 和小鼠 EC9-EC10 的 X 射线晶体结构,其中显示了 EC8-9 典型的钙结合接头,以及改变线性的 EC9-10 无钙接头。 EC重复序列的排列。分子动力学模拟和小角X射线散射实验支持这种非线性构象。模拟还表明,EC9-10 的不弯曲赋予了刚性尖端连杆一定的弹性。新结构提供了原钙粘蛋白 15 的非典型 EC 连接子的第一视角,并表明它们如何在内耳机械转导中发挥作用,这对其他钙粘蛋白具有影响。













































 京公网安备 11010802027423号
京公网安备 11010802027423号