当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transformation of Cyclic Amides and Uracil-Derived Nitrogen Heterocycles During Chlorination
Water Research ( IF 11.4 ) Pub Date : 2025-04-12 , DOI: 10.1016/j.watres.2025.123639
Sungeun Lim, Yufei Wu, William A. Mitch
Water Research ( IF 11.4 ) Pub Date : 2025-04-12 , DOI: 10.1016/j.watres.2025.123639
Sungeun Lim, Yufei Wu, William A. Mitch
|
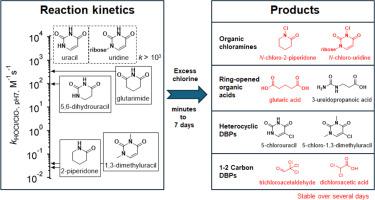
Nitrogen heterocycles are important structural components in biomolecules and anthropogenic chemicals, yet their transformation during chlorine disinfection remains poorly understood. This study investigated chlorination kinetics and product formation for six nitrogen heterocycles of increasing structural complexity, including cyclic amides (2-piperidone, glutarimide, 5,6-dihydrouracil) and uracil derivatives (uracil, uridine, and 1,3-dimethyluracil) to determine how structural variations influence reaction pathways. Apparent second-order rate constants varied widely from 9.2 × 10-3 M-1 s-1 (2-piperidone) to >103 M-1 s-1 (uracil, uridine), largely influenced by the nitrogen pKa values. Chlorination proceeded through initial N-chlorination, forming organic chloramides. While most organic chloramides were transient, that derived from 2-piperidone persisted for days under excess chlorine conditions. For saturated heterocyclic imides (glutarimide, 5,6-dihydrouracil), hydrolysis of the organic chloramides between the imide nitrogen and an adjacent acyl group rapidly formed ring-opened organic acids. Among uracil derivatives, chlorine added across the double bond. For uracil, the resulting 5-chlorouracil rapidly fragmented between the C-4 and C-5 position to release trichloroacetaldehyde at ∼100% yield. Substitution at heterocyclic nitrogens in uridine and 1,3-dimethyluracil limited such fragmentation, forming more stable C-chlorinated heterocyclic or ring-opened products. The reaction patterns observed for these six nitrogen heterocycles were further validated using phthalimide and thymine, demonstrating the broader applicability of the identified reaction trends. These findings enhance our understanding of nitrogen heterocycle chlorination mechanisms and their implications for drinking water disinfection, offering insights into minimizing the formation of potentially harmful DBPs during chlorination.
中文翻译:

氯化过程中环酰胺和尿嘧啶衍生的氮杂环的转化
氮杂环是生物分子和人为化学物质中的重要结构成分,但它们在氯消毒过程中的转化仍然知之甚少。本研究研究了结构复杂性增加的六种氮杂环的氯化动力学和产物形成,包括环酰胺(2-哌啶酮、戊二酰胺、5,6-二氢尿嘧啶)和尿嘧啶衍生物(尿嘧啶、尿苷和 1,3-二甲基尿嘧啶),以确定结构变化如何影响反应途径。表观二阶速率常数从 9.2 × 10-3 M-1 s-1(2-哌啶酮)到 >103 M-1 s-1(尿嘧啶、尿苷)变化很大,主要受氮 pKa 值的影响。氯化通过初始 N-氯化进行,形成有机氯酰胺。虽然大多数有机氮脲是短暂的,但 2-哌啶酮衍生的氮脲在过量的氯条件下会持续数天。对于饱和杂环酰亚胺(戊二酰胺,5,6-二氢尿嘧啶),酰亚胺氮和相邻酰基之间的有机氯酰胺水解迅速形成开环有机酸。在尿嘧啶衍生物中,氯在双键上添加。对于尿嘧啶,所得的 5-氯尿嘧啶在 C-4 和 C-5 位置之间迅速碎裂,以 ∼100% 的产率释放出三氯乙醛。尿苷和 1,3-二甲基尿嘧啶中杂环氮的取代限制了这种碎裂,形成了更稳定的 C-氯化杂环或开环产物。使用邻苯二甲酰亚胺和胸腺嘧啶进一步验证了观察到的这六种氮杂环的反应模式,证明了已确定的反应趋势具有更广泛的适用性。 这些发现增强了我们对氮杂环氯化机制及其对饮用水消毒的影响的理解,为最大限度地减少氯化过程中潜在有害 DBP 的形成提供了见解。
更新日期:2025-04-12
中文翻译:

氯化过程中环酰胺和尿嘧啶衍生的氮杂环的转化
氮杂环是生物分子和人为化学物质中的重要结构成分,但它们在氯消毒过程中的转化仍然知之甚少。本研究研究了结构复杂性增加的六种氮杂环的氯化动力学和产物形成,包括环酰胺(2-哌啶酮、戊二酰胺、5,6-二氢尿嘧啶)和尿嘧啶衍生物(尿嘧啶、尿苷和 1,3-二甲基尿嘧啶),以确定结构变化如何影响反应途径。表观二阶速率常数从 9.2 × 10-3 M-1 s-1(2-哌啶酮)到 >103 M-1 s-1(尿嘧啶、尿苷)变化很大,主要受氮 pKa 值的影响。氯化通过初始 N-氯化进行,形成有机氯酰胺。虽然大多数有机氮脲是短暂的,但 2-哌啶酮衍生的氮脲在过量的氯条件下会持续数天。对于饱和杂环酰亚胺(戊二酰胺,5,6-二氢尿嘧啶),酰亚胺氮和相邻酰基之间的有机氯酰胺水解迅速形成开环有机酸。在尿嘧啶衍生物中,氯在双键上添加。对于尿嘧啶,所得的 5-氯尿嘧啶在 C-4 和 C-5 位置之间迅速碎裂,以 ∼100% 的产率释放出三氯乙醛。尿苷和 1,3-二甲基尿嘧啶中杂环氮的取代限制了这种碎裂,形成了更稳定的 C-氯化杂环或开环产物。使用邻苯二甲酰亚胺和胸腺嘧啶进一步验证了观察到的这六种氮杂环的反应模式,证明了已确定的反应趋势具有更广泛的适用性。 这些发现增强了我们对氮杂环氯化机制及其对饮用水消毒的影响的理解,为最大限度地减少氯化过程中潜在有害 DBP 的形成提供了见解。



































 京公网安备 11010802027423号
京公网安备 11010802027423号