当前位置:
X-MOL 学术
›
J. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Communication: State-to-state dynamics of the Cl + H2O → HCl + OH reaction: Energy flow into reaction coordinate and transition-state control of product energy disposal
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-06-22 13:03:27 , DOI: 10.1063/1.4922650 Bin Zhao 1 , Zhigang Sun 2 , Hua Guo 1
The Journal of Chemical Physics ( IF 3.1 ) Pub Date : 2015-06-22 13:03:27 , DOI: 10.1063/1.4922650 Bin Zhao 1 , Zhigang Sun 2 , Hua Guo 1
Affiliation
|
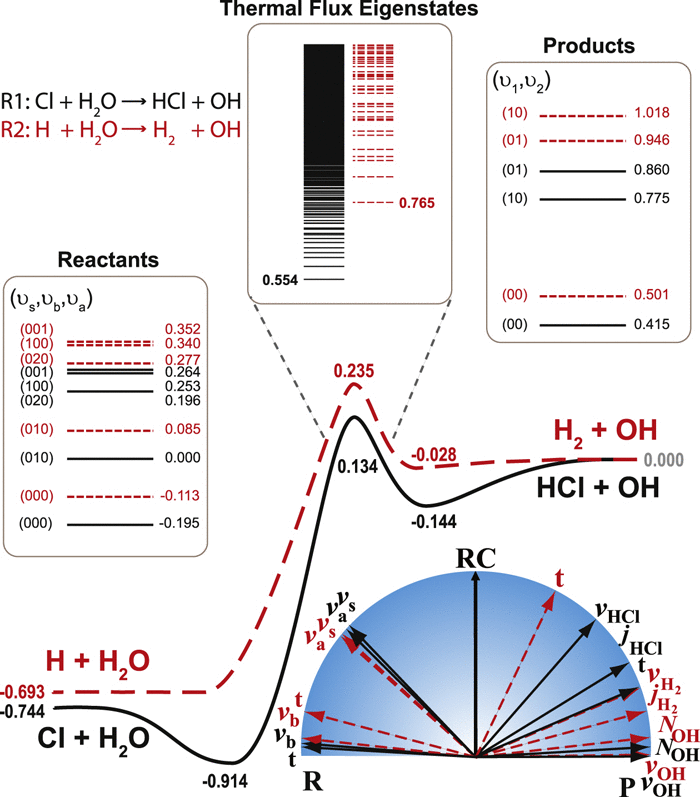
Quantum state-to-state dynamics of a prototypical four-atom reaction, namely, Cl + H2O → HCl + OH, is investigated for the first time in full dimensionality using a transition-state wave packet method. The state-to-state reactivity and its dependence on the reactant internal excitations are analyzed and found to share many similarities both energetically and dynamically with the H + H2O → H2 + OH reaction. The strong enhancement of reactivity by the H2O stretching vibrational excitations in both reactions is attributed to the favorable energy flow into the reaction coordinate near the transition state. On the other hand, the insensitivity of the product state distributions with regard to reactant internal excitation stems apparently from the transition-state control of product energy disposal.
中文翻译:

交流:Cl + H2O→HCl + OH反应的状态动态:能量流入反应坐标和产品能量处置的过渡态控制
使用过渡态波包方法首次以全维度研究了典型的四原子反应,即Cl + H 2 O→HCl + OH的量子态间动力学。分析了状态间的反应性及其对反应物内部激发的依赖性,发现与H + H 2 O→H 2 + OH反应在能量上和动态上都有许多相似之处。H 2强烈增强反应性在两个反应中拉伸振动激发归因于在过渡态附近有利的能量流入反应坐标。另一方面,产物状态分布对反应物内部激发的不敏感性显然源自产物能量处置的过渡态控制。
更新日期:2015-06-23
中文翻译:

交流:Cl + H2O→HCl + OH反应的状态动态:能量流入反应坐标和产品能量处置的过渡态控制
使用过渡态波包方法首次以全维度研究了典型的四原子反应,即Cl + H 2 O→HCl + OH的量子态间动力学。分析了状态间的反应性及其对反应物内部激发的依赖性,发现与H + H 2 O→H 2 + OH反应在能量上和动态上都有许多相似之处。H 2强烈增强反应性在两个反应中拉伸振动激发归因于在过渡态附近有利的能量流入反应坐标。另一方面,产物状态分布对反应物内部激发的不敏感性显然源自产物能量处置的过渡态控制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号