当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyridine-N-oxide catalyzed asymmetric N-acylative desymmetrization of sulfonimidamides
Chemical Science ( IF 7.6 ) Pub Date : 2025-04-08 , DOI: 10.1039/d5sc01270h
Cui-Mei Guo 1 , Fang-Yuan Zhang 1 , Yin Tian 2 , Ming-Sheng Xie 1 , Hai-Ming Guo 1
Chemical Science ( IF 7.6 ) Pub Date : 2025-04-08 , DOI: 10.1039/d5sc01270h
Cui-Mei Guo 1 , Fang-Yuan Zhang 1 , Yin Tian 2 , Ming-Sheng Xie 1 , Hai-Ming Guo 1
Affiliation
|
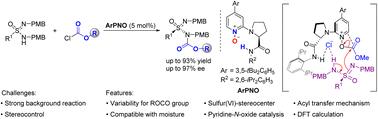
A highly efficient enantioselective N-acylative desymmetrization of sulfonimidamides with chloroformates was reported using chiral 4-arylpyridine-N-oxide as the catalyst, affording N-acylative sulfonimidamides with sulfur(VI)-stereocenters in high yields and excellent enantioselectivities. Experiments and DFT calculations support an acyl transfer mechanism, and the nucleophilic substitution of sulfonimidamide by the O-acyloxypyridinium cation intermediate is the enantio-determining step of the reaction. The reaction features variability for acyloxy groups and compatibility with moisture.
中文翻译:

吡啶-N-氧化物催化磺酰氨基酰胺的不对称 N-酰化去对称化
报道了使用手性 4-芳基吡啶-N-氧化物对磺酰氨基酰胺与氯甲酸酯的高效对映选择性 N-酰化去对称化,以高产率和优异的对映选择性获得具有硫 (VI) -立体中心的 N-酰化磺酰氨基酰胺。实验和 DFT 计算支持酰基转移机制,O-酰氧基吡啶阳离子中间体对磺酰酰胺的亲核取代是反应的对映体决定步骤。该反应具有酰氧基的可变性和与水分的相容性。
更新日期:2025-04-08
中文翻译:

吡啶-N-氧化物催化磺酰氨基酰胺的不对称 N-酰化去对称化
报道了使用手性 4-芳基吡啶-N-氧化物对磺酰氨基酰胺与氯甲酸酯的高效对映选择性 N-酰化去对称化,以高产率和优异的对映选择性获得具有硫 (VI) -立体中心的 N-酰化磺酰氨基酰胺。实验和 DFT 计算支持酰基转移机制,O-酰氧基吡啶阳离子中间体对磺酰酰胺的亲核取代是反应的对映体决定步骤。该反应具有酰氧基的可变性和与水分的相容性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号