当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and Preclinical Evaluation of 7-benzyl-N-(substituted)-pyrrolo[3,2-d]pyrimidin-4-amines as Single Agents with Microtubule Targeting Effects along with Triple-acting Angiokinase Inhibition as Antitumor Agents
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2016-11-17 00:56:38 Roheeth Kumar Pavana, Shruti Choudhary, Anja Bastian, Michael A. Ihnat, Raouli Bai, Ernest Hamel, Aleem Gangjee
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2016-11-17 00:56:38 Roheeth Kumar Pavana, Shruti Choudhary, Anja Bastian, Michael A. Ihnat, Raouli Bai, Ernest Hamel, Aleem Gangjee
|
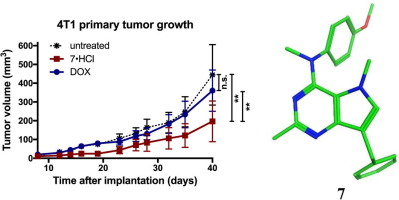
The utility of cytostatic antiangiogenic agents (AA) in cancer chemotherapy lies in their combination with cytotoxic chemotherapeutic agents. Clinical combinations of AA with microtubule targeting agents (MTAs) have been particularly successful. The discovery, synthesis and biological evaluations of a series of 7-benzyl-N-substituted-pyrrolo[3,2-d]pyrimidin-4-amines are reported. Novel compounds which inhibit proangiogenic receptor tyrosine kinases (RTKs) including vascular endothelial growth factor receptor-2 (VEGFR-2), platelet-derived growth factor receptor-β (PDGFR-β) and epidermal growth factor receptor (EGFR), along with microtubule targeting in single molecules are described. These compounds also inhibited blood vessel formation in the chicken chorioallantoic membrane (CAM) assay, and some potently inhibited tubulin assembly (with activity comparable to that of combretastatin A-4 (CA)). In addition, some of the analogs circumvent the most clinically relevant tumor resistance mechanisms (P-glycoprotein and β-III tubulin expression) to microtubule targeting agents (MTA). These MTAs bind at the colchicine site on tubulin. Two analogs displayed two to three digit nanomolar GI50 values across the entire NCI 60 tumor cell panel and one of these, compound 7, freely water soluble as its HCl salt, afforded excellent in vivo antitumor activity against an orthotopic triple negative 4T1 breast cancer model and was superior to doxorubicin.
中文翻译:

7-苄基-N-(取代)-吡咯并[3,2-d]嘧啶-4-胺的单一药物的发现和临床前评价,具有微管靶向作用以及三作用血管激酶抑制作用作为抗肿瘤药物
细胞抑制抗血管生成剂(AA)在癌症化学疗法中的用途在于它们与细胞毒性化学治疗剂的组合。AA与微管靶向剂(MTA)的临床组合特别成功。报道了一系列的7-苄基-N-取代的吡咯并[3,2-d]嘧啶-4-胺的发现,合成和生物学评估。抑制包括血管内皮生长因子受体2(VEGFR-2),血小板衍生生长因子受体β(PDGFR-β)和表皮生长因子受体(EGFR)在内的促血管生成受体酪氨酸激酶(RTK)的新型化合物描述了单分子靶向。这些化合物还可以抑制鸡绒膜尿囊膜(CAM)检测中的血管形成,以及一些有效抑制的微管蛋白组装(其活性可与康美他汀A-4(CA)媲美)。另外,一些类似物绕过了对微管靶向剂(MTA)的最临床相关的肿瘤抵抗机制(P-糖蛋白和β-III微管蛋白表达)。这些MTA在微管蛋白上的秋水仙碱位点结合。两个类似物显示出2到3位数的纳摩尔GI在整个NCI 60肿瘤细胞组中有50个值,其中之一是化合物7(作为HCl盐可自由溶于水),对原位三阴性4T1乳腺癌模型具有出色的体内抗肿瘤活性,优于阿霉素。
更新日期:2016-11-17
中文翻译:

7-苄基-N-(取代)-吡咯并[3,2-d]嘧啶-4-胺的单一药物的发现和临床前评价,具有微管靶向作用以及三作用血管激酶抑制作用作为抗肿瘤药物
细胞抑制抗血管生成剂(AA)在癌症化学疗法中的用途在于它们与细胞毒性化学治疗剂的组合。AA与微管靶向剂(MTA)的临床组合特别成功。报道了一系列的7-苄基-N-取代的吡咯并[3,2-d]嘧啶-4-胺的发现,合成和生物学评估。抑制包括血管内皮生长因子受体2(VEGFR-2),血小板衍生生长因子受体β(PDGFR-β)和表皮生长因子受体(EGFR)在内的促血管生成受体酪氨酸激酶(RTK)的新型化合物描述了单分子靶向。这些化合物还可以抑制鸡绒膜尿囊膜(CAM)检测中的血管形成,以及一些有效抑制的微管蛋白组装(其活性可与康美他汀A-4(CA)媲美)。另外,一些类似物绕过了对微管靶向剂(MTA)的最临床相关的肿瘤抵抗机制(P-糖蛋白和β-III微管蛋白表达)。这些MTA在微管蛋白上的秋水仙碱位点结合。两个类似物显示出2到3位数的纳摩尔GI在整个NCI 60肿瘤细胞组中有50个值,其中之一是化合物7(作为HCl盐可自由溶于水),对原位三阴性4T1乳腺癌模型具有出色的体内抗肿瘤活性,优于阿霉素。






























 京公网安备 11010802027423号
京公网安备 11010802027423号