当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Suspension Electrolytes with Catalytically Self‐Expediating Desolvation Kinetics for Low‐Temperature Zinc Metal Batteries
Advanced Materials ( IF 27.4 ) Pub Date : 2025-03-24 , DOI: 10.1002/adma.202501079
Jing Dong 1, 2 , Xiaomin Cheng 2 , Haifeng Yang 2 , Huihua Li 3, 4 , Haitao Liu 5 , Lujie Jia 2 , Yongzheng Zhang 6 , Qinghua Guan 1, 2 , Jiqiang Jia 7 , Fanglin Wu 3, 4 , Jing Zhang 7 , Meinan Liu 1, 2 , Hongzhen Lin 1, 2 , Jian Wang 3, 4
Advanced Materials ( IF 27.4 ) Pub Date : 2025-03-24 , DOI: 10.1002/adma.202501079
Jing Dong 1, 2 , Xiaomin Cheng 2 , Haifeng Yang 2 , Huihua Li 3, 4 , Haitao Liu 5 , Lujie Jia 2 , Yongzheng Zhang 6 , Qinghua Guan 1, 2 , Jiqiang Jia 7 , Fanglin Wu 3, 4 , Jing Zhang 7 , Meinan Liu 1, 2 , Hongzhen Lin 1, 2 , Jian Wang 3, 4
Affiliation
|
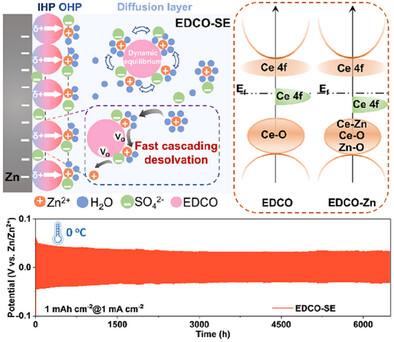
The conventional electrolyte for rechargeable aqueous zinc metal batteries (AZMBs) breeds many problems such as Zn dendrite growth and side reaction of hydrogen evolution reaction, which are fundamentally attributed to the uneven ion flux owing to the high barriers of desolvation and diffusion of Zn[(H2 O)6 ]2+ clusters. Herein, to modulate the [Zn(H2 O)6 ]2+ solvation structure, the suspension electrolyte engineering employed with electron‐delocalized catalytic nanoparticles is initially proposed to expedite desolvation kinetics. As a proof, the electron‐density‐adjustable CeO2‐ x 2 O)6 ]2+ structure. Meanwhile, the defect‐rich CeO2‐ x 2‐ x −2 and an extended lifespan exceeding 6500 h with lower overpotentials of 34 mV under 0 °C. Matched with polyaniline cathodes, the full cells with suspension electrolyte exhibit a capacity‐retention of 96.75% at 1 A g−1 under −20 °C as well as a long lifespan of up to 400 cycles in a large‐areal pouch cell, showcasing promising potentials of suspension electrolyte for practical AZMBs.
中文翻译:

用于低温锌金属电池的具有催化自加速脱溶剂动力学的悬浮电解质
可充电锌金属电池 (AZMBs) 的常规电解质存在 Zn 枝晶生长和析氢反应副反应等许多问题,这些问题从根本上归因于 Zn[(H2O)6]2+ 团簇的脱溶剂化和扩散高势垒导致离子通量不均匀。在此,为了调节 [Zn(H2O)6] 2+ 溶剂化结构,最初提出了采用电子离域催化纳米颗粒的悬浮电解质工程来加速脱溶剂化动力学。作为证明,电子密度可调的 CeO2-x 被引入商业电解质中并优先吸附在 Zn 表面,调节 Zn[(H2O)6]2+ 结构。同时,富含缺陷的 CeO2‐x 重新分配局部空间电场以均匀离子通量动力学并抑制枝晶生长,一系列理论模拟、光谱和实验测量证实了这一点。令人鼓舞的是,CeO2-x 修饰的悬浮电解质在 5 mA cm-2 下可实现 1200 次循环的长期稳定性,使用寿命超过 6500 小时,在 0 °C 下过电位更低,为 34 mV。 与聚苯胺阴极匹配,带有悬浮电解质的全电池在 -20 °C 下在 1 A g-1 下表现出 96.75% 的容量保持率,并且在大面积软包电池中具有长达 400 次循环的长寿命,展示了悬浮电解质在实用 AZMB 中的潜力。
更新日期:2025-03-24
中文翻译:

用于低温锌金属电池的具有催化自加速脱溶剂动力学的悬浮电解质
可充电锌金属电池 (AZMBs) 的常规电解质存在 Zn 枝晶生长和析氢反应副反应等许多问题,这些问题从根本上归因于 Zn[(H2O)6]2+ 团簇的脱溶剂化和扩散高势垒导致离子通量不均匀。在此,为了调节 [Zn(H2O)6] 2+ 溶剂化结构,最初提出了采用电子离域催化纳米颗粒的悬浮电解质工程来加速脱溶剂化动力学。作为证明,电子密度可调的 CeO2-x 被引入商业电解质中并优先吸附在 Zn 表面,调节 Zn[(H2O)6]2+ 结构。同时,富含缺陷的 CeO2‐x 重新分配局部空间电场以均匀离子通量动力学并抑制枝晶生长,一系列理论模拟、光谱和实验测量证实了这一点。令人鼓舞的是,CeO2-x 修饰的悬浮电解质在 5 mA cm-2 下可实现 1200 次循环的长期稳定性,使用寿命超过 6500 小时,在 0 °C 下过电位更低,为 34 mV。 与聚苯胺阴极匹配,带有悬浮电解质的全电池在 -20 °C 下在 1 A g-1 下表现出 96.75% 的容量保持率,并且在大面积软包电池中具有长达 400 次循环的长寿命,展示了悬浮电解质在实用 AZMB 中的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号