当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Blue‐Light‐Induced Stereoselective Synthesis of α‐Alkylated Amino Acid Derivatives
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2025-03-23 , DOI: 10.1002/ejoc.202401477
Hongying Fan 1 , Meiling Ye 1 , Xue Zhang 1 , Jinyu Hou 1 , Liulin Jiao 1 , Jian Chen 1 , Li Guo 1 , Zhong Lian 1 , Yong Wu 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2025-03-23 , DOI: 10.1002/ejoc.202401477
Hongying Fan 1 , Meiling Ye 1 , Xue Zhang 1 , Jinyu Hou 1 , Liulin Jiao 1 , Jian Chen 1 , Li Guo 1 , Zhong Lian 1 , Yong Wu 1
Affiliation
|
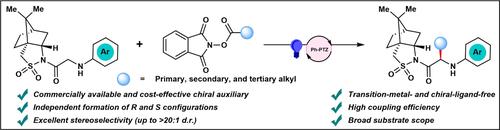
A photoredox‐catalyzed decarboxylative C(sp 3 )−H alkylation of glycine derivatives, utilizing (+)/(−)‐camphorsultam as a chiral auxiliary and alkyl N ‐hydroxyphthalimide (NHP) ester as a radical precursor, has been developed, providing an efficient approach for the stereoselective synthesis of valuable enantioenriched unnatural α ‐alkylated amino acid derivatives. The methodology operates under mild conditions, demonstrates broad substrate tolerance, and exhibits excellent stereoselectivity. Preliminary mechanistic investigations indicate that the reaction proceeds via a radical pathway.
中文翻译:

蓝光诱导的 α-烷基化氨基酸衍生物的立体选择性合成
已经开发了一种光氧化还原催化的甘氨酸衍生物脱羧 C(sp3)-H 烷基化反应,利用 (+)/(-)-樟脑素作为手性助剂和烷基 N-羟基邻苯二甲酰亚胺 (NHP) 酯作为自由基前体,为有价值的对映体富集非天然 α-烷基化氨基酸衍生物的立体选择性合成提供了一种有效的方法。该方法在温和的条件下运行,表现出广泛的底物耐受性,并表现出优异的立体选择性。初步机理研究表明,反应通过自由基途径进行。
更新日期:2025-03-23
中文翻译:

蓝光诱导的 α-烷基化氨基酸衍生物的立体选择性合成
已经开发了一种光氧化还原催化的甘氨酸衍生物脱羧 C(sp3)-H 烷基化反应,利用 (+)/(-)-樟脑素作为手性助剂和烷基 N-羟基邻苯二甲酰亚胺 (NHP) 酯作为自由基前体,为有价值的对映体富集非天然 α-烷基化氨基酸衍生物的立体选择性合成提供了一种有效的方法。该方法在温和的条件下运行,表现出广泛的底物耐受性,并表现出优异的立体选择性。初步机理研究表明,反应通过自由基途径进行。






































 京公网安备 11010802027423号
京公网安备 11010802027423号