当前位置:
X-MOL 学术
›
J. Adv. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mitochondrial fatty acid oxidation as the target for blocking therapy-resistance and inhibiting tumor recurrence: The proof-of-principle model demonstrated for ovarian cancer cells
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2025-03-17 , DOI: 10.1016/j.jare.2025.03.026
Hui Lin 1 , Lingfang Wang 2 , Hanwen Chen 3 , Yuqing Shen 4 , Conghui Wang 5 , Yite Xue 6 , Zhi Zheng 7 , Yanan Zhang 8 , Dajing Xia 9 , Yihua Wu 9 , Fenfen Wang 5 , Xiao Li 5 , Xiaodong Cheng 5 , Hui Wang 10 , Junfen Xu 5 , Weiguo Lu 11
中文翻译:

线粒体脂肪酸氧化作为阻断治疗耐药和抑制肿瘤复发的靶点:卵巢癌细胞的原理验证模型
接受当前化疗和靶向治疗的癌症患者经常达到部分缓解,最终以更具侵袭性、耐药性的肿瘤表型复发。在一定程度上,耐药持久性 (DTP) 细胞负责全身抗癌治疗后残留肿瘤和获得性耐药的发生。因此,迫切需要针对 DTP 细胞的新型疗法来防止耐药和肿瘤复发。
我们旨在研究耐药卵巢癌持久性细胞的特征和关键脆弱性,并寻找潜在的治疗策略。
我们通过将卵巢癌亲本细胞暴露于致死剂量的紫杉醇来构建紫杉醇耐受的卵巢癌持久性细胞。进行蛋白质组学分析、体外和体内测定,以确定可能成为持久性细胞潜在脆弱性的生物过程。
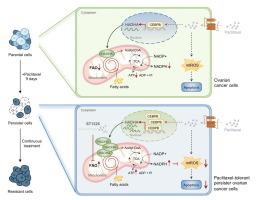
发现紫杉醇耐受卵巢癌持久性细胞通过脂肪酸氧化 (FAO) 的上调进行代谢重编程。用 FAO 抑制剂 ST1326 治疗抑制了 FAO 并增加了持久性细胞对紫杉醇的敏感性。此外,紫杉醇和 ST1326 的联合治疗在卵巢癌复发的小鼠模型中以令人满意的生物安全性阻止了卵巢肿瘤复发,表明 FAO 破坏可以提高基于紫杉醇的治疗在卵巢癌中的疗效。从机制上讲,我们发现紫杉醇治疗上调了 CEBPB,CEBPB 是一种转录因子,可诱导 FAO 相关酶 HADHA 的表达,并导致 FAO 在持久性细胞中升高。
本研究揭示了 FAO 在紫杉醇耐受卵巢癌持续性细胞中的上调,并提供了一种针对持续性细胞的前瞻性紫杉醇-ST1326 联合疗法,可以防止获得性耐药性的发展,并在未来实现卓越的长期卵巢癌控制。我们的研究建立了一个概念框架,用于推进卵巢癌治疗的个性化治疗方法和提高患者预后。
更新日期:2025-03-18
Journal of Advanced Research ( IF 11.4 ) Pub Date : 2025-03-17 , DOI: 10.1016/j.jare.2025.03.026
Hui Lin 1 , Lingfang Wang 2 , Hanwen Chen 3 , Yuqing Shen 4 , Conghui Wang 5 , Yite Xue 6 , Zhi Zheng 7 , Yanan Zhang 8 , Dajing Xia 9 , Yihua Wu 9 , Fenfen Wang 5 , Xiao Li 5 , Xiaodong Cheng 5 , Hui Wang 10 , Junfen Xu 5 , Weiguo Lu 11
Affiliation
|
Introduction
Cancer patients treated with current chemotherapeutic and targeted therapies frequently achieve partial remission, which ultimately relapse with more aggressive, drug-resistant tumor phenotypes. To a certain extent, drug-tolerant persister (DTP) cells are responsible for residual tumors after systemic anticancer therapy and the onset of acquired drug resistance. Therefore, novel therapies targeting DTP cells to prevent drug resistance and tumor recurrence are urgently needed.Objectives
We aimed to investigate the traits and key vulnerabilities of drug-tolerant ovarian cancer persister cells and to seek out potential therapeutic strategies.Methods
We constructed paclitaxel-tolerant ovarian cancer persister cells by exposing ovarian cancer parental cells to a lethal dose of paclitaxel. Proteomics analysis, in vitro and in vivo assays were performed to identify biological processes that could serve as potential vulnerabilities in persister cells.Results
Paclitaxel-tolerant ovarian cancer persister cells were found to undergo a metabolic reprogramming through the upregulation of fatty acid oxidation (FAO). Treatment with the FAO inhibitor ST1326 suppressed FAO and increased sensitivity to paclitaxel in persister cells. Moreover, combination therapy with paclitaxel and ST1326 prevented ovarian tumor recurrence with satisfactory biosafety in a mouse model of ovarian cancer relapse, indicating that FAO disruption can improve the efficacy of paclitaxel-based therapy in ovarian cancer. Mechanistically, we found that paclitaxel treatment upregulated CEBPB, a transcription factor that induced the expression of the FAO-related enzyme HADHA and contributed to FAO elevation in persister cells.Conclusions
This study revealed an upregulation of FAO in paclitaxel-tolerant ovarian cancer persister cells and provided a prospective paclitaxel-ST1326 combination therapy targeting persister cells that may prevent the development of acquired drug resistance and achieve superior long-term ovarian cancer control in the future. Our research established a conceptual framework for advancing personalized treatment approaches and enhancing patient outcomes in ovarian cancer therapy.中文翻译:

线粒体脂肪酸氧化作为阻断治疗耐药和抑制肿瘤复发的靶点:卵巢癌细胞的原理验证模型
介绍
接受当前化疗和靶向治疗的癌症患者经常达到部分缓解,最终以更具侵袭性、耐药性的肿瘤表型复发。在一定程度上,耐药持久性 (DTP) 细胞负责全身抗癌治疗后残留肿瘤和获得性耐药的发生。因此,迫切需要针对 DTP 细胞的新型疗法来防止耐药和肿瘤复发。
目标
我们旨在研究耐药卵巢癌持久性细胞的特征和关键脆弱性,并寻找潜在的治疗策略。
方法
我们通过将卵巢癌亲本细胞暴露于致死剂量的紫杉醇来构建紫杉醇耐受的卵巢癌持久性细胞。进行蛋白质组学分析、体外和体内测定,以确定可能成为持久性细胞潜在脆弱性的生物过程。
结果
发现紫杉醇耐受卵巢癌持久性细胞通过脂肪酸氧化 (FAO) 的上调进行代谢重编程。用 FAO 抑制剂 ST1326 治疗抑制了 FAO 并增加了持久性细胞对紫杉醇的敏感性。此外,紫杉醇和 ST1326 的联合治疗在卵巢癌复发的小鼠模型中以令人满意的生物安全性阻止了卵巢肿瘤复发,表明 FAO 破坏可以提高基于紫杉醇的治疗在卵巢癌中的疗效。从机制上讲,我们发现紫杉醇治疗上调了 CEBPB,CEBPB 是一种转录因子,可诱导 FAO 相关酶 HADHA 的表达,并导致 FAO 在持久性细胞中升高。
结论
本研究揭示了 FAO 在紫杉醇耐受卵巢癌持续性细胞中的上调,并提供了一种针对持续性细胞的前瞻性紫杉醇-ST1326 联合疗法,可以防止获得性耐药性的发展,并在未来实现卓越的长期卵巢癌控制。我们的研究建立了一个概念框架,用于推进卵巢癌治疗的个性化治疗方法和提高患者预后。

































 京公网安备 11010802027423号
京公网安备 11010802027423号