当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the NS2B-NS3 protease from Zika virus after self-cleavage.
Nature Communications ( IF 14.7 ) Pub Date : 2016-11-15 , DOI: 10.1038/ncomms13410
Wint Wint Phoo , Yan Li , Zhenzhen Zhang , Michelle Yueqi Lee , Ying Ru Loh , Yaw Bia Tan , Elizabeth Yihui Ng , Julien Lescar , CongBao Kang , Dahai Luo
Nature Communications ( IF 14.7 ) Pub Date : 2016-11-15 , DOI: 10.1038/ncomms13410
Wint Wint Phoo , Yan Li , Zhenzhen Zhang , Michelle Yueqi Lee , Ying Ru Loh , Yaw Bia Tan , Elizabeth Yihui Ng , Julien Lescar , CongBao Kang , Dahai Luo
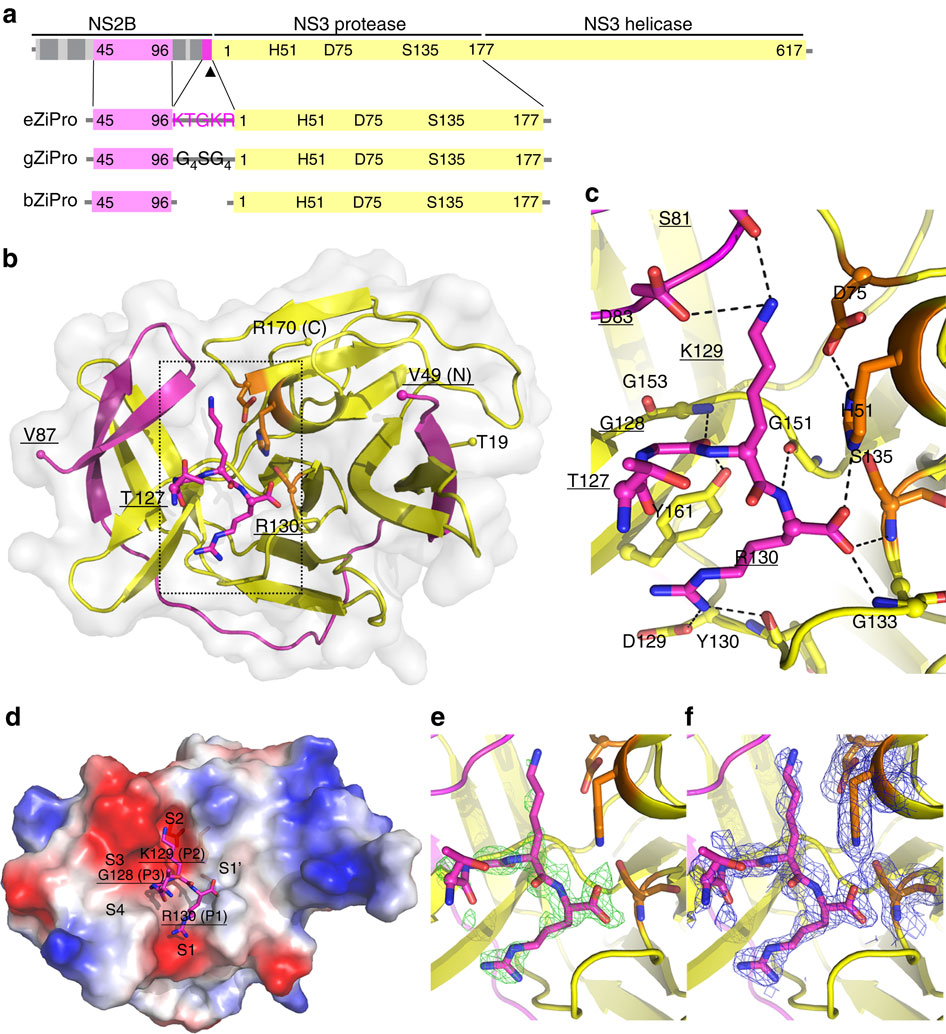
|
The recent outbreak of Zika virus (ZIKV) infections in the Americas represents a serious threat to the global public health. The viral protease that processes viral polyproteins during infection appears as an attractive drug target. Here we report a crystal structure at 1.84 Å resolution of ZIKV non-structural protein NS2B-NS3 protease with the last four amino acids of the NS2B cofactor bound at the NS3 active site. This structure represents a post-proteolysis state of the enzyme during viral polyprotein processing and provides insights into peptide substrate recognition by the protease. Nuclear magnetic resonance (NMR) studies and protease activity assays unravel the protein dynamics upon binding the protease inhibitor BPTI in solution and confirm this finding. The structural and functional insights of the ZIKV protease presented here should advance our current understanding of flavivirus replication and accelerate structure-based antiviral drug discovery against ZIKV.
中文翻译:

自切割后寨卡病毒的NS2B-NS3蛋白酶的结构。
最近在美洲爆发的寨卡病毒(ZIKV)感染对全球公共健康构成了严重威胁。在感染过程中加工病毒多蛋白的病毒蛋白酶似乎是有吸引力的药物靶标。在这里,我们报告了ZIKV非结构蛋白NS2B-NS3蛋白酶的1.84Å分辨率的晶体结构,其中NS2B辅因子的最后四个氨基酸结合在NS3活性位点上。这种结构代表了病毒多蛋白加工过程中酶的蛋白水解后状态,并提供了对蛋白酶识别肽底物的了解。在溶液中结合蛋白酶抑制剂BPTI时,核磁共振(NMR)研究和蛋白酶活性测定揭示了蛋白质动力学,并证实了这一发现。
更新日期:2016-11-17
中文翻译:

自切割后寨卡病毒的NS2B-NS3蛋白酶的结构。
最近在美洲爆发的寨卡病毒(ZIKV)感染对全球公共健康构成了严重威胁。在感染过程中加工病毒多蛋白的病毒蛋白酶似乎是有吸引力的药物靶标。在这里,我们报告了ZIKV非结构蛋白NS2B-NS3蛋白酶的1.84Å分辨率的晶体结构,其中NS2B辅因子的最后四个氨基酸结合在NS3活性位点上。这种结构代表了病毒多蛋白加工过程中酶的蛋白水解后状态,并提供了对蛋白酶识别肽底物的了解。在溶液中结合蛋白酶抑制剂BPTI时,核磁共振(NMR)研究和蛋白酶活性测定揭示了蛋白质动力学,并证实了这一发现。

































 京公网安备 11010802027423号
京公网安备 11010802027423号