当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
[5+1] Annulation of aza-o-Quinone Methides with Primary Amines: Construction of 3,4-Dihydroquinazolin-2(1H)-One Derivatives
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2025-03-13 , DOI: 10.1002/ejoc.202500267
Gang Wang 1 , Hao-Bin Liu 1 , Zhao-Lin He 2
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2025-03-13 , DOI: 10.1002/ejoc.202500267
Gang Wang 1 , Hao-Bin Liu 1 , Zhao-Lin He 2
Affiliation
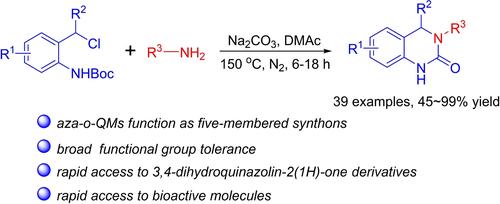
|
A [5+1] annulation reaction between in situ generated aza-o-quinone methides and primary amines has been successfully realized. This approach facilitates the efficient synthesis of a diverse range of 3,4-dihydroquinazolin-2(1H)-one derivatives, exhibiting excellent functional group compatibility. Moreover, the utility of this methodology is further demonstrated by its application in the synthesis of a potential CDK5 inhibitor, a biologically active compound.
中文翻译:

[5+1]氮杂-邻醌二酯与伯胺的环化:3,4-二氢喹唑啉-2(1H)-酮衍生物的构建
原位生成的氮杂-邻醌均化物与伯胺之间的 [5+1] 环化反应已成功实现。该方法有助于高效合成多种 3,4-二氢喹唑啉-2(1H)-酮衍生物,表现出优异的官能团相容性。此外,该方法在合成潜在的 CDK5 抑制剂(一种生物活性化合物)中的应用进一步证明了该方法的实用性。
更新日期:2025-03-13
中文翻译:

[5+1]氮杂-邻醌二酯与伯胺的环化:3,4-二氢喹唑啉-2(1H)-酮衍生物的构建
原位生成的氮杂-邻醌均化物与伯胺之间的 [5+1] 环化反应已成功实现。该方法有助于高效合成多种 3,4-二氢喹唑啉-2(1H)-酮衍生物,表现出优异的官能团相容性。此外,该方法在合成潜在的 CDK5 抑制剂(一种生物活性化合物)中的应用进一步证明了该方法的实用性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号